Translate this page into:
Nutritional Vitamin D deficiency rickets in children – Challenges in diagnosis, management, and prevention
*Corresponding author: Raja Padidela, Department of Paediatric Endocrinology, Royal Manchester Children’s Hospital, Manchester, United Kingdom. raja.padidela@mft.nhs.uk
-
Received: ,
Accepted: ,
How to cite this article: Dabas A, Padidela R. Nutritional Vitamin D deficiency rickets in children – Challenges in diagnosis, management, and prevention. Wadia J Women Child Health. 2024;3(1):15-24. doi: 10.25259/WJWCH_39_2023
Abstract
Nutritional rickets are characterized by under mineralization of the skeleton that leads to bone deformities and poor growth. The balance between Vitamin D and calcium intake is critical for the maintenance of bone health. A few risk factors that contribute to a high prevalence of rickets in India include poor maternal nutrition, poor dietary calcium, and Vitamin D intake and poor sunshine practices. Early features of rickets may be asymptomatic and may be missed without biochemical and radiological investigations. Severe rickets may be the first manifestation of an underlying non-nutritional rickets that may be misdiagnosed in the absence of a complete workup. The treatment of rickets requires Vitamin D therapy with adequate calcium supplementation. The schedule of treatment with Vitamin D is not standardized, but daily therapy is preferable compared to weekly/monthly stoss therapy. Both cholecalciferol and ergocalciferol may be used for treatment as they are efficacious and cost-effective instead of active Vitamin D preparations. Periodic monitoring for the resolution of biochemical deficiency and improvement in skeletal changes should be emphasized. Prolonged treatment with Vitamin D and calcium should be avoided for the risk of Vitamin D toxicity and nephrocalcinosis. An impetus is required toward the prevention of Vitamin D deficiency. At present, nutritional strategies should focus on a life-cycle approach during the antenatal period, early infancy, and childhood and adolescence. Food fortification is likely to be an effective option, but the efficacy and logistics of this in the Indian setting will require further research.
Keywords
Hypocalcemia
Rickets
Cholecalciferol
Fortification
INTRODUCTION
Rickets continues to be a common nutritional deficiency disease in India. Rickets can result from a deficiency of calcium and/or Vitamin D (calcipenic) or phosphorus (phosphopenic). Vitamin D and parathyroid hormone (PTH) are the principal hormones that control calcium homeostasis [Figure 1]. Inadequate body stores of calcium due to Vitamin D and/or dietary calcium deficiency leads to calcipenic rickets. Vitamin D, PTH, and fibroblast growth factor 23 (FGF23) control phosphorus homeostasis. Phosphopenic rickets usually occurs in metabolic bone disorders that reduce body stores of phosphorus either due to excess FGF23 or tubular leak of phosphorus.[1]
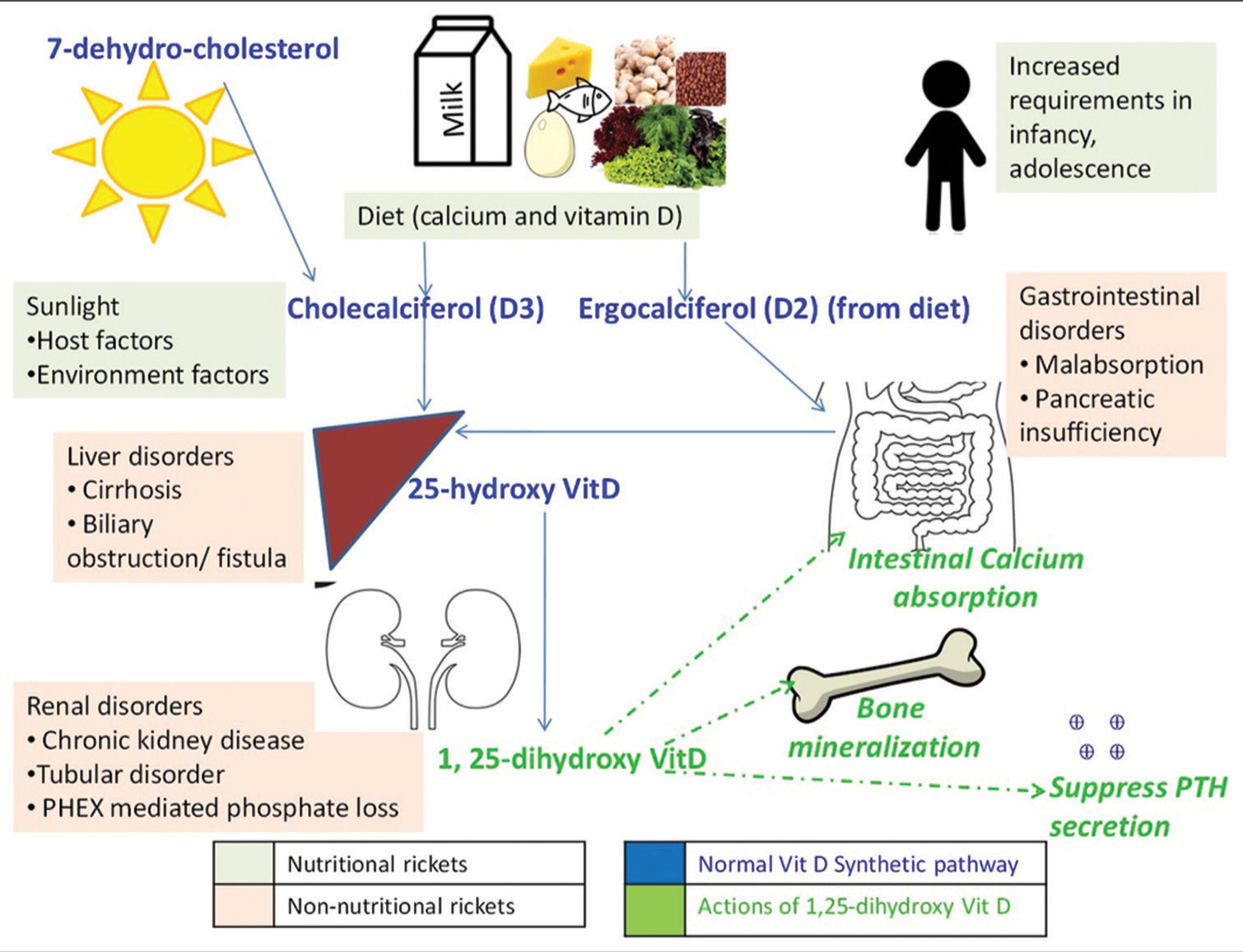
- Normal Vitamin D metabolism and etiology of rickets. The normal biosynthetic pathway of Vitamin D is shown in Blue font with actions of active Vitamin D-1,25-dihydroxy Vitamin D in green; etiology of nutritional rickets (grey box) and non-nutritional rickets (pink box) can be deciphered. PTH: Parathyroid hormone, PHEX: Phosphate regulating endopeptidase X - linked.
Despite an abundance of sunlight, Vitamin D deficiency (VDD) has been reported in Indian children across different studies.[1,2] The nationwide comprehensive national nutrition survey found the prevalence of VDD as 14% among children aged 1–4 years, 18% among school-age children (5–9 years), and 24% among adolescents (10–19 years); higher among girls than boys in adolescence.[3] Likewise, dietary intake of calcium-containing diet continues to be low in Indian children and adolescents which predispose them to nutritional deficiency rickets and osteomalacia.[4] The maternal-child dyad unit also represents an at-risk unit for VDD. The maternal requirements for calcium increase during pregnancy; inadequate dietary intake of calcium and failure of adequate sunlight exposure; and/or Vitamin D supplementation can lead to low maternal and fetal stores of calcium, increasing the risk of infantile hypocalcemia, rickets, and poor postnatal growth.
PATHOPHYSIOLOGY
In calcipenic rickets, reduced body stores of calcium cause secondary hyperparathyroidism. High PTH concentration normalizes calcium levels by increasing the conversion of Vitamin D to active Vitamin D (thereby increasing dietary absorption of calcium) and by mobilization of calcium from the bones. Elevated PTH concentration, however, excretes phosphate in the urine causing hypophosphatemia. Low extracellular fluid phosphate causes the failure of apoptosis of hypertrophic chondrocytes and rickets. Early stages of rickets are, therefore, characterized by normal serum calcium, high PTH, elevated alkaline phosphatase (ALP), and low serum phosphate. Low serum phosphate and elevated ALP concentrations are therefore a hallmark of both calcipenic and phosphopenic rickets.
To some extent, a high intake of dietary calcium can compensate for VDD and vice versa. Most cases of rickets and osteomalacia are therefore due to a combination of dietary calcium deficiency and VDD. A classic example of rickets is seen in a Vitamin D-deficient infant who is exclusively breastfed beyond 6 months of age where reduced calcium concentration of breast milk is unable to supply enough calcium for a growing skeleton.
DIAGNOSIS
The diagnosis of rickets is usually based on clinical findings. Infants present with subtle skeletal signs such as frontal bossing and craniotabes (softening of the skull bones). Early changes in rickets may manifest as non-specific complaints with only associated biochemical changes in the absence of overt radiological changes. These may include generalized lethargy, fatigue, and non-specific muscle or bone pains. Often, isolated biochemical deficiency may be detectable during screening for other systemic conditions such as dermatitis, asthma, or pneumonia.[5,6]
Hypocalcemic seizures may be the hallmark of severe calcium and/or VDD in infants. Older children usually have signs of a skeletal deformity such as short stature, genu valgum/varum, wrist widening, and rachitic rosary (beading at costochondral junctions). Non-ambulatory infants have upper limb changes of rickets and ambulation brings about lower limb changes of rickets. Mild genu varum is physiological till 2 years of age and should be assessed by a physician if it is of parental concern. Older adolescents may sometimes first present with hypocalcemic tetany (thumb adduction, metacarpophalangeal joint flexion, and interphalangeal joint extension). Severe hypocalcemia can manifest as seizures, laryngomalacia/laryngospasm, and cardiomyopathy.[1] Most children with rickets also manifest motor weakness due to proximal myopathy. Severe rickets may present with delay in motor milestones due to myopathy.
It is of paramount importance to evaluate the dietary history in children who present with hypocalcemia for instituting a management plan. The food frequency questionnaire is a quicker and more reliable method of documenting dietary calcium intake and can be useful in children.[7]
A detailed history and examination should be done to ascertain the cause of rickets, as shown in Table 1. As a paradox, a child with severe protein-energy malnutrition will rarely develop signs of rickets as nutrition deprivation prevents bone growth. If a child with rickets has malnutrition or failure to thrive, a differential diagnosis of non-nutritional rickets should always be considered. Anthropometry is a useful tool to assess short stature seen in rickets. With the involvement of long bones in rickets, the upper: lower segment ratio becomes higher and disproportionate.
| History and examination |
Clinical pointers |
|---|---|
| Antenatal and Birth history | • Maternal intake of supplemental calcium during pregnancy and lactation |
| • Birth weight – Small for gestation age and preterm babies can have early-onset hypocalcemia as they require supplemental calcium and Vitamin D after birth. |
|
| Feeding and dietary history | • History of breastfeeding/top-feeds – Breast milk is a poor source of Vitamin D |
| • Use of Vitamin D supplements – Dose and compliance to daily supplementation to be checked | |
| • Complementary feeding – Milk-based diets are poor source of Vitamin D. The quantity and quality of top-milk to be noted (to check for calcium content). Dietary diversity for other micro-nutrients to be checked. | |
| • Use of fortified foods – milk, oil, and dairy products | |
| Developmental history | • History of development in all domains – gross motor, fine motor, socioadaptive, and language – children with rickets usually have only isolated delay in motor milestones. |
| • Global developmental delay may be seen in systemic diseases (like lysosomal storage disorders that may have skeletal changes with organomegaly) | |
| Family history | • History of any other family members with skeletal disorders, alopecia, and dental abnormalities. Prefer to examine parents and siblings to rule out any short stature or bony deformities (clues to non-nutritional rickets, skeletal dysplasia) |
| Systemic complaints in nutritional rickets | • Irritability and poor feeding (infancy) |
| • Weakness, tiredness, and back pain | |
| • Bony deformity | |
| • Abnormal gait (waddling due to myopathy) | |
| • Not growing well in stature | |
| • Seizures – when associated with hypocalcemia | |
| • Tetany – usually seen in older children and adolescents | |
| Systemic complaints that suggest non-nutritional causes | • Weight loss – failure to thrive may suggest a systemic organic cause |
| • Frequent loose stools, weight loss suggesting malabsorption (seen in Celiac disease, IBD) | |
| • Jaundice – suggests chronic liver failure as a systemic cause of rickets | |
| • Fractures – may be rarely seen in severe rickets, but advisable to screen for non-nutritional causes of rickets (like renal tubular acidosis) | |
| • Drug history for drug-induced osteoporosis with corticosteroids, anticonvulsants, calcineurin inhibitors, and cyclosporine | |
| General physical examination | • Anthropometry – short stature, upper segment to lower segment proportion (disproportionate in rickets) |
| • Skull and spine – craniotabes (softening of skull bones), wide open anterior fontanelle and lumbar lordosis in rickets. Craniosynostosis may be seen in metabolic bone diseases like hypophosphatasia or XLH. Alopecia suggests VDDR-type II (lack of Vitamin D receptor activity within keratinocytes) | |
| • Dentition – dental caries, delayed tooth eruption, and enamel hypoplasia in rickets. Dental abscess suggest XLH | |
| • Hair – alopecia may indicate non-nutritional causes like VDDR-II | |
| • Skeletal limb signs – wrist widening, genu valgum, genu varum, and windswept deformity | |
| • Anemia – may be with concurrent nutritional deficiency, or may be with underlying lymphoreticular proliferation in systemic diseases (storage disorders) | |
| • Icterus – suggests underlying hepatic cause such as storage diseases and Wilson disease | |
| • Skin – Xerosis in chronic liver disease, dry scaly skin for concomitant hypothyroidism in polyendocrinopathy, café-au-lait spots in fibrous dysplasia | |
| • Gait – waddling in presence of myopathy with Vitamin D deficiency or phosphate deficiency | |
| Systemic examination | • Cardiac – may detect dilated cardiomyopathy in long standing hypocalcemia or Vitamin D deficiency, arrhythmia with hypocalcemia (prolonged QTc interval on electrocardiogram) |
| • Abdomen – hepatosplenomegaly as part of visceroptosis in rickets, may also be present in other systemic disorders such as Wilson disease or tyrosinemia | |
| • Respiratory – Harrison sulcus due to flail chest wall | |
| • Neurological – increased neuronal excitability seen as Chvostek sign (facial twitching in response to gentle tapping on face) or Trousseau sign (carpopedal spasm after elevated cuff pressure using a sphygmomanometer) |
IBD: Inflammatory bowel disease, VDDR: Vitamin D dependent rickets, XLH: X lined hypophosphatemiaMonitoring
INVESTIGATIONS
The first investigation to be considered in a child with rickets is a metabolic bone profile that includes serum calcium, phosphorus, ALP, serum 25-hydroxy Vitamin D (25-OHD), and PTH. Serum calcium levels indicate total calcium levels, so should be corrected for serum albumin in cases of hypoalbuminemia. Ionized calcium level is not affected by serum globulins or binding proteins and is a more sensitive measure, useful in titrating treatment in response to low or high serum calcium. Serum phosphate levels should ideally be measured in the morning in fasting states. For young children, a minimum of 2–4 h fast without consumption of milk is suggested to measure phosphate levels accurately.
Serum ALP is an excellent marker for diagnosis and monitoring of therapy.[8] Care should be taken to interpret the ALP levels with age-based cutoffs as ALP levels are higher in children and adolescents compared to adults. ALP levels are usually higher in calcipenic rickets than phosphopenic rickets.[9] Serum PTH is a sensitive marker of disturbance in the calcium milieu. PTH levels are raised in rickets (secondary hyperparathyroidism) due to associated dietary calcium deficiency or VDD. The raised PTH levels result in renal phosphate loss through sodium-dependent phosphate co-transporters (NaPi-2a,2b,2c) that cause hypophosphatemia. However, the degree of hypophosphatemia is milder than in phosphopenic rickets. PTH levels begin to decrease following treatment and indicate a therapeutic response but may not return to normal baseline levels at the end of treatment.[10,11] While hypophosphatemia and hyperphosphatemia are hallmarks of both calcipenic and phosphopenic rickets, PTH is elevated only in calcipenic rickets while it is normal or marginally elevated in phosphopenic rickets. Table 2 shows the role of biochemical tests in differentiating the metabolic bone conditions that may mimic nutritional rickets. A raised phosphorus level with signs of rickets could suggest an underlying chronic renal failure that mandates a blood urea and serum creatinine measurement. On occasion, chronic dietary calcium deficiency can present with PTH resistance-like state and is accompanied by elevated phosphorus levels, a biochemical picture similar to pseudohypoparathyroidism. The presence of metabolic acidosis with a normal anion gap suggests a renal tubular disorder that should be confirmed with further investigations.
| Serum calcium | Serum phosphorus | Serum ALP | Serum 25-OHD | Serum PTH | Remarks | |
|---|---|---|---|---|---|---|
| NR | Low | Low/low- normal | Raised | Low | Raised | |
| VDDR-I | Low | Normal/low | Raised | Normal | Raised | Low 1, 25, dihydroxy Vitamin D |
| VDDR-II | Normal/low | Normal Low | Raised | Normal | Raised | Normal 1, 25, dihydroxy Vitamin D |
| XLHR/ADHR/ARHR | Normal | Low | Raised | Normal | Normal | Increased urinary phosphate excretion. Increased FGF23 levels |
| HHRH | Normal | Low | Raised | Normal | Normal | Increased urinary calcium excretion with low-normal phosphate excretion |
NR: Nutritional rickets, VDDR: Vitamin D dependent rickets, XLHR: X-linked hypophosphatemic rickets, ADHR: Autosomal dominant hypophosphatemic rickets, ARHR: Autosomal recessive hypophosphatemic rickets, HHRH: Hereditary hypophosphatemic rickets with hypercalciuria, ALP: Alkaline phosphatase, 25-OHD: 25-hydroxy Vitamin D, PTH: Parathyroid hormone, FGF23: Fibroblast growth factor 23
Serum 25-OHD is measured as the total Vitamin D level (D2 and D3) routinely. Clinically, there is no relevance of separately measuring Vitamin D2/D3 fraction. Hence, a measured serum 25-OHD gives a good estimate of the body’s Vitamin D stores. The optimum cutoffs for Vitamin D have been defined as per the minimum levels required for optimum bone health. The Indian Academy of Pediatrics (2021) and the Global Consensus for Management of nutritional rickets define VDD as serum 25-OHD below 12 ng/mL, insufficiency as 12–20 ng/mL and sufficiency as >20 ng/mL.[5,6] Serum 25-OHD levels are low in rickets, and the degree of VDD predicts the severity of rickets. The treatment, however, remains the same, irrespective of the degree of VDD. Likewise, measurement of an active form of Vitamin D (1,25-OHD) is not required for diagnosis or management of nutritional rickets. At presentation, if the clinical diagnosis of nutritional rickets is not clear, then the sample should be stored for measurement of 1,25-OHD (Vitamin D resistant rickets) and fibroblast growth factor-23 or FGF23 (hypophosphatemic rickets).[10]
The radiological signs of rickets are discernible at the growing ends of long bones. These can be detected in distal radius and ulna on wrist X-ray in upper limb. Similar changes can be seen in lower end of femur and upper tibial physis on X ray of the knee. The classical changes include generalized osteopenia, subperiosteal bone resorption, widening (splaying), cupping, and fraying that lead to expansion of the growth plate. These changes take time to develop and thus may be lacking in early infancy. The long bones may develop deformities with long- standing disease. The degree of radiological changes is usually more severe in calcipenic than in phosphopenic rickets.[1] Typically, these changes tend to improve with treatment and can be objectively measured using the radiological rickets severity score that assigns a score to the wrist (0–4) and knee (0–6); a maximum score of 10 where a higher score indicates worse disease.[12] The beginning of mineralization in the unmineralized metaphyseal ends is visible as a white healing line after 4–6 weeks of therapy. A failure of the healing line to appear in combination with the failure of reduction of ALP and PTH mandates a work-up for the non-nutritional etiology of rickets.
The radiographs also provide clues for non-nutritional causes of skeletal deformities. A list of radiological pointers of disorders other than nutritional rickets is mentioned in Table 3.
| Signs | Inference |
|---|---|
| Metaphyseal or epiphyseal flaying without any irregularity or fraying | Metaphyseal-epiphyseal dysplasia |
| Multiple long bone fractures, healing at variable ages | Osteogenesis imperfecta, child abuse |
| Osteosclerosis and hyperostosis | Autosomal recessive-hypophosphatemic rickets |
| Osteosclerosis with tongue of radiolucency within metaphyses | Hypophosphatasia |
MANAGEMENT
The management of nutritional rickets is essentially based on correcting the calcium deficiency state and normalizing the metabolic bone profile, the maintenance of which over weeks and months leads to radiographic signs of improvement of rickets and osteomalacia. All children being treated for rickets need supplemental calcium at a dose of 50 mg/kg/day up to a maximum of 500 mg/day, irrespective of serum calcium levels.[5,6] This can be met through inexpensive calcium supplements such as syrup or tablets. However, patients should be advised carefully regarding the timing of calcium intake after food to avoid gastrointestinal side effects and to avoid simultaneous consumption of iron supplements. It is important to undertake dietary assessment and encourage intake of calcium-rich products such as dairy products, vegetables, and nuts to improve the calcium deficiency state and prevent the future development of rickets and osteomalacia. Children who present with acute symptomatic hypocalcemia such as seizures, arrhythmia, and cardiomyopathy require normalization of serum calcium levels by intravenous calcium over 24–48 h. A concomitant magnesium deficiency should be evaluated and corrected with oral or intramuscular magnesium.
Vitamin D treatment should be commenced in all children with nutritional rickets. The meta-data from Indian studies suggests that treatment should be instituted at serum 25-OHD level <12 ng/mL as there is an increase in serum PTH below this level.[13,14] However, an optimum level is required in children with any high-risk conditions such as malabsorption, chronic kidney disease, chronic liver disease, primary and secondary osteoporosis, neuromuscular disability, cerebral palsy, or on prolonged duration of drugs such as corticosteroids or immunosuppressants. These children should be treated to maintain serum 25-OHD levels of >20 ng/mL and may require daily supplementation.[5,6]
Vitamin D treatment has been tried in different doses and regimes. The initial regimen consisted of bolus intramuscular dosing of 6 lac IU of cholecalciferol. However, this dose was deemed too high as it could potentially cause Vitamin D toxicity with hypercalcemia and hypercalciuria. The intramuscular route is now no longer recommended for the treatment of nutritional rickets and is reserved for cases where there is a suspicion of malabsorption.[5,6]
Stoss therapy with a high bolus dose of Vitamin D ranging from 100,000 to 300,000 units can be administered to correct deficiency, especially in those where adherence to medication is of concern. This should be followed up with a supplementation dose to prevent the recurrence of the deficiency state. However, there are concerns about bolus doses being less physiological in achieving bone healing. A higher peak rise in serum 25-OHD can increase its inactivation which, would prevent adequate healing of rickets and osteomalacia. The most physiological regimen is, therefore daily administration of Vitamin D in equivalent doses,[11,14,15] as shown in Table 4.[5,6] However, there may exist a wide variation in the treatment practices of rickets in India. In a recent questionnaire-based study on residents and pediatricians by Meshram et al., the median Vitamin D knowledge score was 10 (maximum value 14) with 51% scoring between 7 and 10.[16] The formulation readily available in India is available as 400 IU or 800 IU per mL solution which is inconvenient in volume and is expensive to administer at 2000–6000 IU daily as treatment doses. Newer Vitamin D formulations in India will be required to allow high-dose daily treatment schedules to be made feasible. The treatment algorithm is shown in Figure 2.
| Age | Daily dose for 12 wk* | Alternative intermittent dose regimen, IU | Maintenance dose#(daily) |
|---|---|---|---|
| <6 months | 2000 IU | NA | 400 |
| 6–12 months | 2000 IU | Equivalent of 2000 IU/day may be given on monthly or weekly basis | 400 |
| >12 months | 3000 IU | 60000 IU fortnightly (after every 2 weeks)×5 doses | 600 |
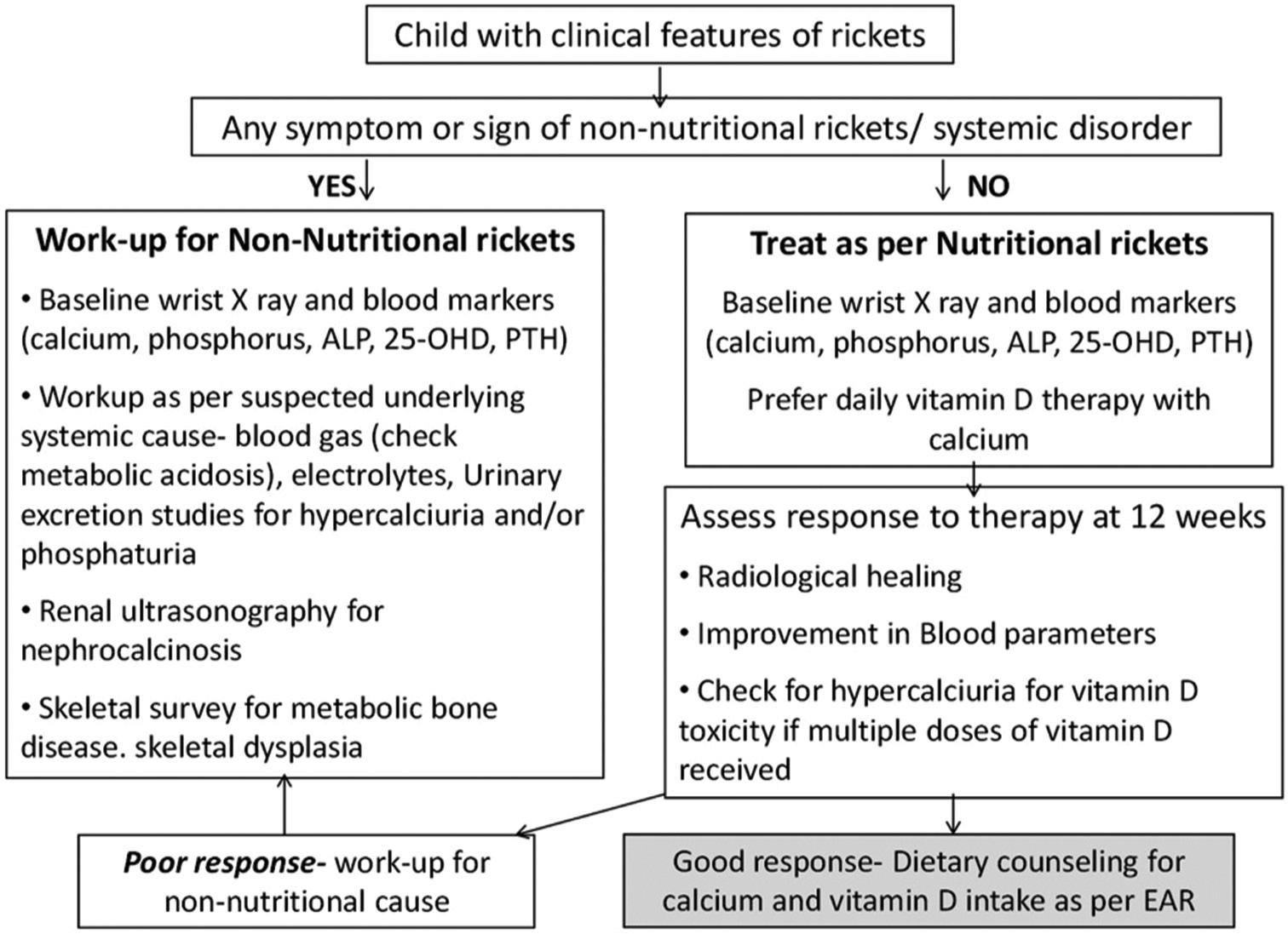
- Treatment algorithm of rickets. ALP: Alkaline phosphatase, EAR: Estimated average requirement, OHD: Hydroxy vitamin D, PTH: Parathyroid hormone.
Treatment of nutritional rickets should be using Vitamin D as cholecalciferol (D3) or ergocalciferol (D2). The use of active forms of Vitamin D such as alpha calcidiol or calcitriol is not recommended for nutritional rickets. It is important to check the constituents of commonly available calcium/Vitamin D formulations to avoid this error.[17] The choice between cholecalciferol or ergocalciferol for therapeutic use shows the superior efficacy of D3 than D2 in increasing serum 25-OHD levels and reducing PTH levels.[18] In addition, the limited availability of ergocalciferol preparations restricts its common use.
Monitoring
A good healing response can be seen in the first few weeks of therapy as a subjective improvement in symptoms such as malaise and weakness. The biochemical improvement follows and among different parameters, serum ALP is a sensitive marker that shows a decline. Further, an increase in serum calcium and 25-OHD levels with a decrease in PTH (secondary hyperparathyroidism) is seen. However, almost a quarter of children with nutritional rickets may fail to normalize the ALP or PTH at the end of 12 weeks and may need prolongation of therapy ensuring adequate calcium and Vitamin D maintenance doses.[11] In a questionnaire-based study at a tertiary level institute in India, 32% of pediatricians/residents monitored for radiological healing, 15.6% by serum calcium, phosphate, and ALP, and 11.4% used a combination of these.[16]
Any child who demonstrates a worsening of biochemical parameters should be investigated for a non-nutritional cause. Likewise, the appearance of similar symptoms or radiological features of rickets with normal biochemistry should also alert for an underlying metabolic bone disorder that could be masked by accompanying nutritional VDD or calcium deficiency.[10]
Vitamin D toxicity is a possibility if inadvertently multiple high doses of Vitamin D have been administered, especially if multiple physicians are involved in the treatment. Hypercalcemia and hypercalciuria should be monitored and if found to be high, then renal ultrasound should be performed to detect nephrocalcinosis.
Radiological monitoring for mineralization is a time-tested objective marker of healing. The earliest sign that appears can be seen as a radiological sclerotic line at the metaphyseal ends by 4 weeks. This is followed by improvement in osteopenia and bone remodeling with a reduction in fraying or splaying of metaphyseal ends and a reduction in the rickets severity score. A score of 1.5 or below indicates an ongoing process of healing and may be achieved at the end of 12 weeks,[12] though a few children may take longer.
CHALLENGES
The diagnosis and management of rickets is straightforward if appropriate history, clinical examination and investigations are undertaken; yet, it has its own challenges in India. The clinical presentation is usually late when significant skeletal deformities are visible. The complete workup of biochemical and radiological investigations at serial intervals is constrained in smaller health facilities. The biochemical assays need standardization with proper collection and transport of samples. A lapse in the standard operating procedures can give erroneous results that do not aid in the diagnosis or management.
The nutritional trend in India by National Nutrition Monitoring Bureau from 1975 to 2017 showed that only 37% of households in India had daily consumption of >70% recommended daily allowance (RDA) of calcium with 44% of households having <50% RDA consumption.[4] Similarly, a study on 220 schoolchildren from Pune showed >75% of children and adolescents consumed diets insufficient for RDA of calcium.[19] The high amount of phytate in the Indian diet also contributes to poor dietary calcium. The presence of cow milk allergy may limit the intake of dairy products during childhood. The marginally low calcium intake is usually unable to meet the increased demands during periods of rapid growth putting infants, toddlers, and adolescents at an increased risk of rickets and osteomalacia. Failure to address dietary intake of calcium can lead to poor healing of rickets or frequent relapses.
A standardized schedule for the management of nutritional rickets with Vitamin D and calcium supplementation is a global challenge. A plethora of protocols, however, provide an opportunity to use a schedule tailor-made for the patient taking into consideration their unique clinical and social circumstances. Concern over the bioavailability of Vitamin D formulations has been raised in the past. The recent availability of micellized water-soluble Vitamin D preparations obviates the issue of bioavailability as seen with fat-soluble preparations. However, cost remains a concern with the use of water-soluble micellized Vitamin D preparations.
Children with rickets may be lost to follow-up till the completion of therapy. The families may also seek multiple second opinions, in a short duration of time, with repeated treatment schedules which can lead to Vitamin D toxicity. There is a delay and unawareness in diagnosing nonnutritional rickets and limited access to second-line investigations in resource-limited facilities. This leads to the risk of hypervitaminosis and adds to the skeletal morbidity of the undiagnosed bone disorder.
PREVENTION
Vitamin D conversion in the skin is the predominant source of Vitamin D and requires adequate sun exposure with no environmental interference. The clinical evidence on the role of sunlight in improving Vitamin D status in the Indian population suggests a sunlight duration of 17–30 min in infants and 30–45 min in older children over 15–40% of body surface area.[6]
Calcium deficiency should be prevented by encouraging an intake of calcium throughout childhood by intake of dairy products, eggs, green leafy vegetables, and calcium-rich grains like millets. The year 2023 has been marked as the year of millets to promote the consumption of millets which are rich in fiber and micronutrients. The RDA for calcium suggested is 400–600 mg/day in 1–9-year-old and from 600 to 800 mg/day in adolescents.[20] Likewise, adequate calcium and Vitamin D intake should be reinforced during pregnancy and lactation to optimize both maternal and infant bone health.[21] Variable schedules and doses of maternal Vitamin D supplementation have been tested and found effective in preventing VDD in the infant, but a minimum of 600 IU as an estimated average requirement (EAR) of Vitamin D should be met by the mother.[5,6]
However, the same strategy may be ineffective to achieve the EAR of Vitamin D as most dietary sources are poor sources of Vitamin D. Ergocalciferol (plant source) is absorbed well but is a weaker source of Vitamin D. The amount of D3 present in milk is in low quantity and further lower in skimmed or semi-skimmed milk which is insufficient to meet the EAR. Data from the NHANES survey (2015–2018) showed that children of all ages consumed less than EAR of 10 μg/day.[22] Vitamin D3 is present in a few animal sources such as fatty fish like salmon, tuna, and mackerel, but their consumption is usually not in amounts sufficient as EAR in Indian children.
Fortification is the process of enrichment or addition of micronutrients to the food in one-third of the total RDA such that consumption of fortified substances does not lead to intoxication with regular consumption of foods. The common foodstuffs used as vehicles for Vitamin D fortification are dairy products, fruit juices, ready-to-eat foods, infant foods, cereals, wheat flour, bread, and oil. The added costs of milk fortification are meager 2 paise/L milk. Fortification of different food products (most commonly milk) with different quantities of Vitamin D has been tested and shown to be effective.[23] There is early Indian experience with Vitamin D fortification in Indian children.[24] A recent efficacy analysis of Vitamin D2 fortified milk consumption for 3 months in school children showed that a dose of 240 IU Vitamin D2/day was ineffective in preventing VDD during the winter season.[25] The recent summary based on NHANES data suggests providing Vitamin-D-fortified foods and higher Vitamin D fortification levels in school meals to achieve EAR of Vitamin D during childhood.[22] The Food Safety Standards Authority of India recommends the use of cholecalciferol or ergocalciferol for fortification in India using a fat-soluble premix which is added to the milk.[26] The rise in serum 25-OHD levels is higher with D3 than with D2. The EAR for Vitamin D is set as 400–600 IU (10–15 ug) for Indian children.[20] Mandatory fortification is recommended when the prevalence of VDD is 20% or the prevalence of rickets is >1%. Accordingly, mandatory fortification is a required strategy for the Indian setting, which will need food and milk production to be centralized and industrialized.[27]
Vitamin D supplementation has been advised in high-risk groups and during infancy to meet the EAR (400 IU during infancy)[5,6] and found effective when used in higher doses.[13] A comparative study reported superior efficacy of supplementation than sunlight exposure during infancy, proving it a reliable source for the prevention of VDD. [28] Vitamin D supplementation circumvents the logistic and efficacy issues with fortification. It is more effective than fortification as per the change in serum 25-OHD levels achieved. However, when we plan to compare the effectiveness of a universal supplementation or fortification program, fortification seems to be a more pragmatic solution. [27] Around 20% of fortification costs would cover the costs of food control, monitoring, and public education. The cost of supplements, monitoring their intake for overdose, adherence especially in high-risk groups, and mass outreach are a few concerns, that may be negated with fortification.
Calcium fortification has been suggested for countries with low baseline calcium intakes using a vehicle that is safe, frequently consumed by the population, remains stable on storage and does not affect the bioavailability of absorption of other nutrients like iron.[29] Grains (wheat, rice, and maize) are commonly fortified with calcium. The implementation of mandatory calcium fortification of flour had issues of limited centralized production (30–100%) in a few countries as in India, where local production for consumption of wheat is common. The consumption of cereals and millets was more than 70% of daily intake in the majority (>65%) of households in the NNMB report,[4] making it perhaps a good vehicle for calcium fortification.
CONCLUSION
The control of nutritional deficiency disorders requires an inclusive approach toward the child and the family that addresses the dietary diversity and sociocultural milieu. Prevention of VDD should focus on a healthy diet and lifestyle (outdoor activities) to ensure optimum mineral acquisition during periods of rapid growth. At present, food fortification is in its nascent stages in India but may prove to be a feasible solution on a mass scale. The treatment of rickets needs to be monitored biochemically and radiologically for response, and early referral should be sought in incomplete response for non-nutritional causes of rickets. The most appropriate cutoff of serum 25-OHD level to be achieved in apparently healthy childhood populations may still be considered contentious, but consensus suggests a level >20 ng/mL is sufficient for maintaining bone health.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Systematic review on vitamin D level in healthy Indian population and analysis of its associated factors. Indian J Endocrinol Metab. 2017;21:765-75.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D status and determinants in Indian children and adolescents: A multicentre study. Sci Rep. 2022;12:16790.
- [CrossRef] [PubMed] [Google Scholar]
- Comprehensive national nutrition survey (CNNS) 2016-2018. Available from: https://nhm.gov.in/writereaddata/l892s/1405796031571201348.pdf [Last accessed on 2020 Mar 09]
- [Google Scholar]
- Modern India and the tale of twin nutrient deficiency-calcium and vitamin D-nutrition trend data 50 years-retrospect, introspect, and prospect. Front Endocrinol (Lausanne). 2019;10:493.
- [CrossRef] [PubMed] [Google Scholar]
- Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394-415.
- [CrossRef] [PubMed] [Google Scholar]
- Indian Academy of Pediatrics Revised (2021) guidelines on prevention and treatment of vitamin D deficiency and rickets. Indian Pediatr. 2022;59:142-58.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid assessment of dietary calcium intake. Arch Dis Child. 2016;101:634-6.
- [CrossRef] [PubMed] [Google Scholar]
- Alkaline phosphatase in pediatric patients with genu varum caused by vitamin D-deficient rickets. Endocr J. 2021;68:807-15.
- [CrossRef] [PubMed] [Google Scholar]
- Alkaline phosphatase in clinical practice in childhood: Focus on rickets. Front Endocrinol (Lausanne). 2023;14:1111445.
- [CrossRef] [PubMed] [Google Scholar]
- Rickets guidance: Part II-management. Pediatr Nephrol. 2022;37:2289-302.
- [CrossRef] [Google Scholar]
- Daily v. weekly oral vitamin D3 therapy for nutritional rickets in Indian children: A randomized controlled open-label trial. Br J Nutr 2022:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Radiographic scoring method for the assessment of the severity of nutritional rickets. J Trop Pediatr. 2000;46:132-9.
- [CrossRef] [PubMed] [Google Scholar]
- Supplementation with three different daily doses of vitamin D3 in healthy pre-pubertal schoolgirls: A cluster randomized trial. Indian Pediatr. 2018;55:951-6.
- [CrossRef] [PubMed] [Google Scholar]
- Daily vs. monthly oral vitamin D3 for treatment of symptomatic vitamin D deficiency in infants: A randomized controlled trial. J Pediatr Endocrinol Metab. 2023;36:683-91.
- [CrossRef] [PubMed] [Google Scholar]
- Low dose depot oral vitamin D3v. daily oral vitamin D3 for treating nutritional rickets: A randomised clinical trial. Br J Nutr 2021:1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Study of knowledge, attitude, and practices of child care physicians toward nutritional vitamin D deficiency in pediatric population. Wadia J Women Child Health. 2023;2:3-9.
- [CrossRef] [Google Scholar]
- Price dispersion of vitamin D supplements over time: An initiative for prescriber education. Indian J Endocrinol Metab. 2021;25:142-7.
- [CrossRef] [PubMed] [Google Scholar]
- Relative efficacy of vitamin D2 and vitamin D3 in improving vitamin D status: Systematic review and meta-analysis. Nutrients. 2021;13:3328.
- [CrossRef] [PubMed] [Google Scholar]
- Determinants of vitamin D status in Indian school children. Indian J Endocrinol Metab. 2018;22:244-8.
- [CrossRef] [PubMed] [Google Scholar]
- National Institute of Nutrition. Indian Council of Medical Research. Available from: http://ninindia.org/DietaryGuidelinesforNINwebsite.pdf [Last accessed on 2021 Oct 03]
- [Google Scholar]
- Effect of maternal supplementation with two different doses of vitamin D during lactation on vitamin D status, anthropometry and bone mass of infants: A randomized controlled trial. Indian Pediatr. 2022;59:276-82.
- [CrossRef] [PubMed] [Google Scholar]
- Perspective: School meal programs require higher vitamin D fortification levels in milk products and plant-based alternatives-evidence from the national health and nutrition examination surveys (NHANES 2001-2018) Adv Nutr. 2022;13:1440-9.
- [CrossRef] [PubMed] [Google Scholar]
- Vitamin D food fortification and nutritional status in children: A systematic review of randomized controlled trials. Nutrients. 2019;11:2766.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of vitamin D fortified milk supplementation on vitamin D status of healthy school children aged 10-14 years [Published correction appears in Osteoporos Int 2014;25:1655] Osteoporos Int. 2013;24:2335-43.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of daily supplementation of milk fortified with vitamin D2 for three months in healthy school children: A randomized placebo controlled trial. Indian Pediatr. 2021;58:820-5.
- [CrossRef] [PubMed] [Google Scholar]
- Standards of fortification of standard foods. 2018. Ministry of Health and Family Welfare, Government of India. Available from: https://archive.fssai.gov.in/home/fss-legislation/notifications/gazette-notification.html [Last accessed on 2020 Mar 09]
- [Google Scholar]
- The burden of vitamin D deficiency in Indian children: The time is right for vitamin D food fortification. Indian Pediatr. 2023;60:181-2.
- [CrossRef] [PubMed] [Google Scholar]
- Sunlight exposure vs oral vitamin D supplementation for prevention of vitamin D deficiency in infancy: A randomized controlled trial. Indian Pediatr. 2022;59:852-8.
- [CrossRef] [PubMed] [Google Scholar]
- Regulatory and policy-related aspects of calcium fortification of foods. Implications for implementing national strategies of calcium fortification. Nutrients. 2020;12:1022.
- [CrossRef] [PubMed] [Google Scholar]






