Translate this page into:
Fatal disseminated congenital cytomegalovirus infection in a twin preterm neonate: A clinical-pathological diagnosis
*Corresponding author: R. R. Prashanth, Department of Neonatology, Seth GS Medical College and KEM Hospital, Mumbai, Maharashtra, India. prash2635@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Patel T, Sathe P, Singh S, Prashanth RR, Nair S, Haribalakrishna A. Fatal disseminated congenital cytomegalovirus infection in a twin preterm neonate: A clinical-pathological diagnosis. Wadia J Women Child Health. 2024;3:99-103. doi: 10.25259/WJWCH_32_2024
Abstract
We report on a rare case of congenital cytomegalovirus (CMV) in a dichorionic diamniotic twins. The uniqueness of one of the twins having a fatal disseminated CMV infection with the other twin being normal has been discussed. In cases with no antenatal diagnosis, the role of post-clinical-pathological correlation using fetal autopsy has been described with fetal autopsy images (gross and microscopy). The possible immunological basis for the selective affection of one twin and a review of literature on similar cases have also been done.
Keywords
Cytomegalovirus
Twin
Owl eye inclusion
Preterm
Neonate
INTRODUCTION
Congenital cytomegalovirus (cCMV) infection has an overall prevalence of 0.3–2.2% and is the most common intrauterine infection. However, only 23 such cases have been reported in twin pregnancies to date.[1] Preterm infants and infants with primary immune disorders of T cells and natural killer cells are at a greater risk of mortality from cCMV infection.[2]
CASE REPORT
A 1600 g female neonate was born to a primigravida as the first of dichorionic diamniotic (DCDA) twins following in vitro fertilization, with donor egg and self-sperm. The cause of infertility in the mother was attributed to primary ovarian insufficiency. Antenatal sonography confirmed twin pregnancy; she had a regular antenatal follow-up until 20 weeks of gestation with no apparent obstetric or medical complications till then. She was referred to our institution with spontaneous onset preterm labor at 32 weeks of gestation, during which there was fetal distress in one of the twins leading to an emergency cesarean section.
The first twin was a female, weighed 1425 g, and cried immediately after birth. The second twin, also a female, the index neonate, weighing 1600 g (appropriate for gestational age) did not cry at birth and had an appearance, pulse, grimace, activity, and respiration score of 3/10 and 4/10. She required extensive resuscitation including intubation, chest compression, and injection of adrenaline.
On examination in the delivery room, she had several purple-colored disseminated flat lesions over the face, trunk, and lower limbs measuring 1–3 mm which did not blanch on pressure [Figure 1]. There was gross hepatomegaly measuring 6 cm below the right costal margin, firm in consistency, and splenomegaly measuring 4 cm below the left costal margin.
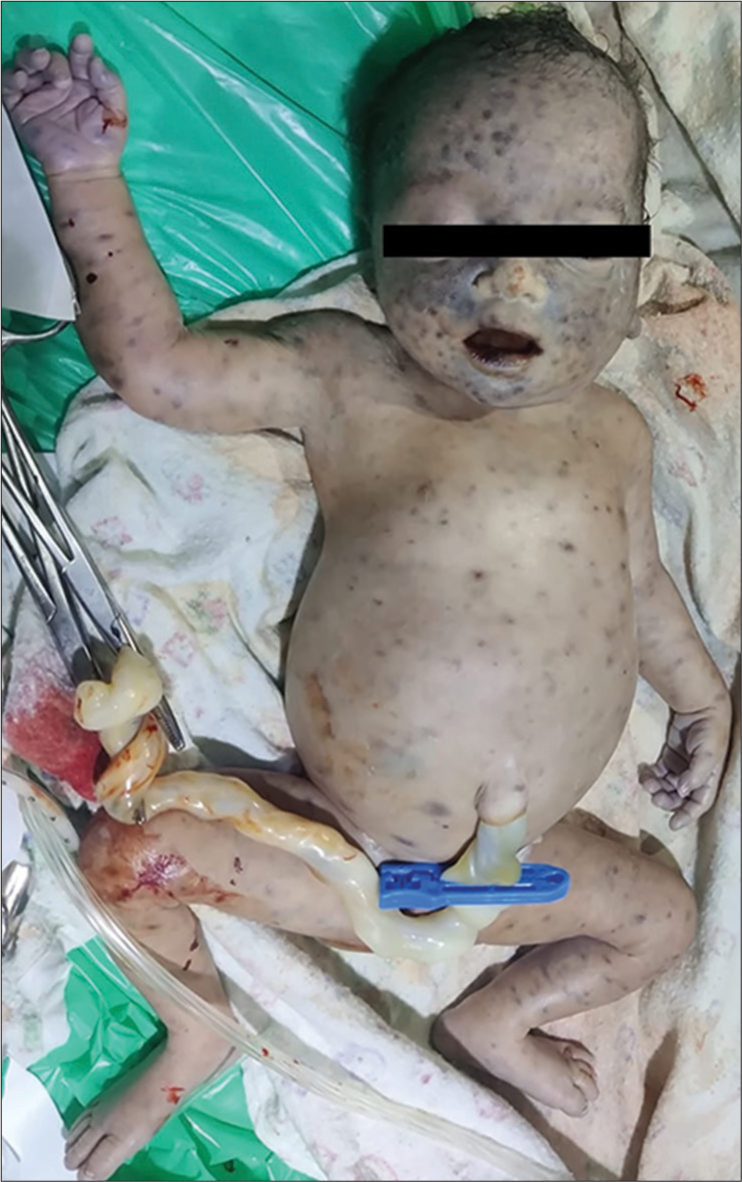
- Delivery room examination showing blueberry muffin spots and hepatosplenomegaly.
Other anthropometric measurements were normal as per the modified Fenton’s chart – head circumference: 29 cm (50–95th centile) and length: 40 cm (50–95th centile).
There was no evidence of generalized edema, pleural effusion, or any suggestion of hydrops fetalis. There was no dysmorphism, cataract, with the rest of the systemic examination being normal.
The placenta weighed 800 g and was DCDA with a normal gross appearance individually weighing 400 g each. The examination of the other twin was normal.
The differential diagnosis that was considered included congenital intrauterine infection: TORCH (congenital rubella and cCMV), congenital leukemia cutis, neuroblastoma, rhabdomyosarcoma, and Langerhans cell histiocytosis.
The index neonate following extensive resuscitation developed refractory shock and severe hypoxic ischemic encephalopathy with multiorgan dysfunction and succumbed at 1 h of life. Consent of parents for postmortem was obtained and findings of the post-mortem evaluation of the index neonate are summarized in Table 1 [Figures 2-4].
| Autopsy findings | ||
|---|---|---|
| Organ | Gross | Microscopy |
| Skin | Diffuse and purple-colored flat lesions all over the body | Hematopoietic cells in the dermis and subcutaneous tissue |
| Liver | Gross hepatomegaly [Figure 2] | Portal triaditis with multiple foci of extramedullary hematopoiesis The bile ductules and hepatocytes show intracytoplasmic and intranuclear inclusions with a typical “Owl eye” appearance [Figure 3] |
| Heart, Lung, Kidney, Pancreas | Mildly enlarged, Normal, Normal, Normal | “Owl eye inclusions” are seen in the tubular epithelium of the kidney, lung alveolar epithelium, and the acini of the pancreas with inflammatory reactions around them |
| Placenta | Mildly enlarged | Chronic villitis with no inclusion bodies [Figure 4] |
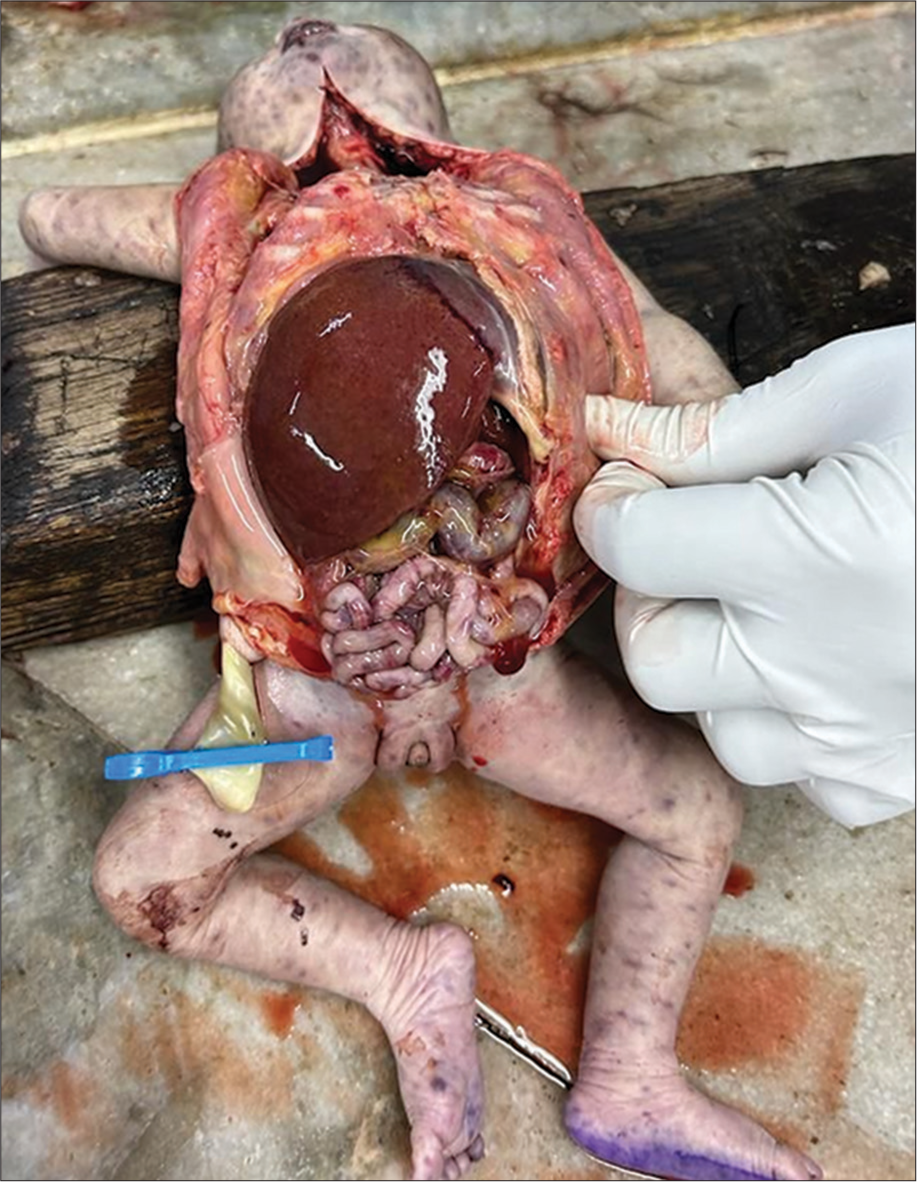
- Fetal autopsy confirming gross hepatosplenomegaly.
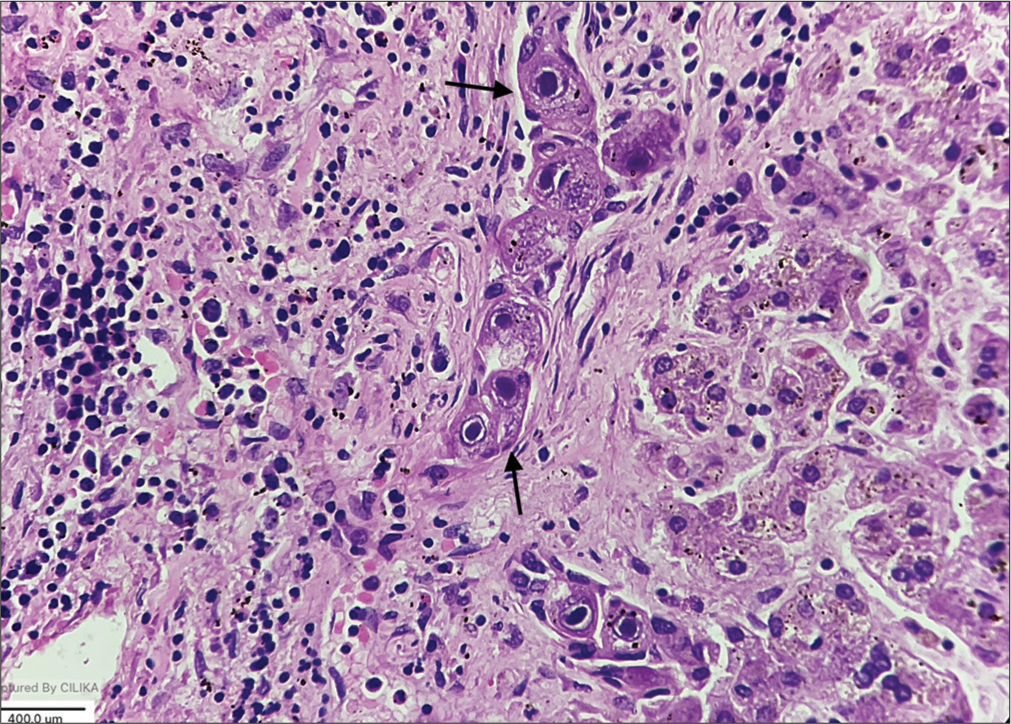
- Bile duct epithelium shows cytomegaly and the characteristic “owl eye” intranuclear inclusions (black arrows) (Hematoxylin and eosin (HE), × 400).
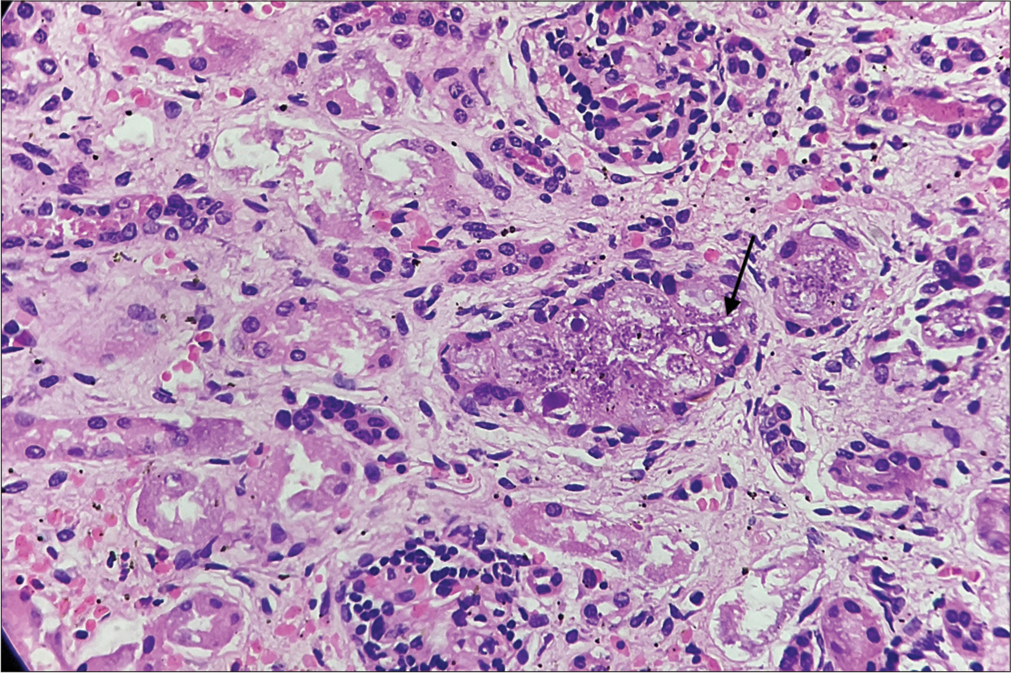
- Renal tubular epithelium shows cytomegaly and the characteristic “owl eye” intranuclear inclusions (black arrow) (Hematoxylin and eosin (HE), ×400).
The history of the mother was revisited. There was no history of fever or rash contact with flu-like symptoms. Antenatal sonography until 20-week anomaly scan was normal and no scan for detection of any growth lag or soft markers was available after that. The complete blood count of the deceased neonate was normal, and the postnatal evaluation of the mother and other normal twins is summarized in Table 2. No other investigations for CMV confirmation including serology, urine, or ultrasonography could be done in the index neonate.
| Laboratory investigation | Mother | Other twin |
|---|---|---|
| CMV IgM | Negative | Negative |
| CMV IgG | >180 | >180 |
| CMV PCR | Negative | Negative |
CMV: Cytomegalovirus, IgM: Immunoglobulin M, IgG: Immunoglobulin G, PCR: Polymerase chain reaction
The cause of death in the index neonate was attributed to a disseminated cCMV. The other twin is currently 3 months old and continues to be normal with normal hearing and vision.
Final diagnosis: Disseminated fatal cCMV infection in a DCDA twin.
DISCUSSION
Moderate to severely symptomatic congenital infection is characterized by hepatosplenomegaly and cutaneous manifestation in 40–60%.[1] These cutaneous manifestations include blueberry muffin spots which represent abnormal postnatal expression of fetal extramedullary dermal hematopoiesis persisting beyond the 5th month of gestation. These are often characterized by multiple purpura, red to violaceous macules, papules, or nodules.[3]
The selective severe affection of one of the twins leading to neonatal death, as in the index case makes this congenital infection more unique. Four such cases have been reported to date, of which three died in utero.[4] Primary infection during pregnancy has a higher risk of vertical transmission compared to recurrent infection (32% vs. 1.4%).[5] Primary infection refers to the acquisition of CMV infection for the 1st time during pregnancy without any pre-existing immunity, whereas non-primary infection refers to the reactivation of previous infection in pregnant women with preformed CMV antibodies or acquiring a different CMV strain. The severity of the malformation due to congenital infection is the highest when the primary CMV infection occurs in the first/second trimester, that is, during organogenesis and neuronal migration with anti-CMV antibodies playing a crucial role in the prevention of infection.[6]
Immunoglobulin-G (IgG) and immunoglobulin-M (IgM) against CMV antigens are often used for identifying primary maternal CMV infection. In those with positive IgM, IgG avidity testing should be used. At present, there is no recommendation for universal CMV infection during pregnancy and it is reserved for those with unexplained flu-like illness or fetuses showing sonographic features. To confirm fetal CMV infection, real-time polymerase chain reaction of amniotic fluid has the highest yield when done after 20 weeks of gestation and at least 6 weeks from the time of maternal infection.[6,7] In this index case, the mother remained completely asymptomatic, and antenatal ultrasonograms until 20 weeks were normal and her CMV serology workup done 1 week after the delivery of the affected neonate showed negative CMV deoxyribonucleic acid titers with predominantly IgG and absent IgM response. However, in the absence of IgG avidity testing, it was probably an old infection which the mother might be exposed to the infection in the second or third trimester.
The index neonate is premature and born at 32 weeks of gestation. The mortality risk is higher in neonates with immature/abnormal T cells and natural killer function which occurs secondary to prematurity or in those with underlying primary immune disease. In these neonates, CMV viremia causes fatal multisystem involvement leading to pneumonia, thrombocytopenia, and risk of bacterial co-infections. However, central nervous system manifestations including microcephaly and intracranial calcifications are more often seen in term neonates.[8]
Premature neonates <32 weeks’ gestation with symptomatic cCMV are more likely to have pneumonitis, signs of viral sepsis, thrombocytopenia, and co-infections and less likely to have microcephaly or intracranial calcifications than term neonates.[2]
Neonatal immune response is delayed in responding to CMV infection. Various immunopathological factors enhance the risk of cCMV infection in a neonate more so in a preterm neonate. These include (i) lack of typical CMV Th1 signature and predominant Th2 response, (ii) reduced cytolytic activity of Th1 cells and decreased interleukin (IL) 2 productions in response to CMV antigen, and (iii) the Th2 response is characterized by lower levels of interferon (IFN)-Gamma and higher IL-8 levels which indirectly augments CMV replication.[2,9]
These above mechanisms seem to be exemplified in dizygous twin pregnancy with current studies suggesting a hypothesis for selective affection of a single twin with the sparing of the other. These include (i) higher maternal transfer of protective antibodies for the normal twin, (ii) selective hypo functioning of the CD4 cells with lesser antibody response, (iii) lack of typical CMV CD8 signature, (iv) lower levels of IFN-gamma and higher IL8 levels, and (v) lack of fusion of placenta which would enable the transfer of CMV antigen.[10]
Role of autopsy/clinicopathological correlates
The clinical differential diagnosis of a “blueberry muffin rash” can range from infectious causes such as TORCH and other viral infections to neoplasms such as leukemia, neuroblastoma, and rhabdomyosarcoma. Autopsy findings in this case were vital in clinching the diagnosis. While neoplastic mass lesions were not found at autopsy, the peripheral smear also did not show evidence of leukemia. Instead, there was a leukoerythroblastic reaction with an abundance of erythroblasts. Organs such as the liver, kidney, pancreas, and lung showed extramedullary hematopoiesis along with the characteristic inclusions of CMV thus ruling other etiologies.
CONCLUSION
Blueberry muffin spots with hepatosplenomegaly in a preterm neonate even in an asymptomatic mother should raise suspicion for cCMV infection and require workup for the same. As in the index, even in a DCDA twin pregnancy, there might be selective affection for a twin with the other twin being completely normal. In those with fatal intrauterine infection, a postmortem autopsy and clinicopathological correlation help in confirming the diagnosis.
The presence of owl eye inclusion bodies distributed in the liver, kidney, lung, and pancreas and the presence of extramedullary hematopoiesis in the skin is pathognomonic of cCMV infection with blueberry muffin spots.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Prevalence of congenital cytomegalovirus infection in symptomatic newborns under 3 weeks in Tehran, Iran. BMC Infect Dis. 2017;17:688.
- [CrossRef] [PubMed] [Google Scholar]
- Blueberry muffin baby with Cytomegalovirus hepatitis. Indian Dermatol Online J. 2019;10:327-9.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital cytomegalovirus infection among twin pairs. J Matern Fetal Neonatal Med. 2016;29:3439-44.
- [CrossRef] [PubMed] [Google Scholar]
- Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253-76.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17:e177-88.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomegalovirus infection in pregnancy-counselling challenges in the setting of generalised testing. Maedica (Bucur). 2020;15:253-7.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence and impact of CMV infection in very low birth weight infants. Pediatrics. 2014;133:e609-15.
- [CrossRef] [PubMed] [Google Scholar]
- Immune responses to congenital cytomegalovirus infection. Microbes Infect. 2018;20:543-51.
- [CrossRef] [PubMed] [Google Scholar]
- Differential congenital cytomegalovirus infection in dichorionic diamniotic twins-A case report and literature review. IDCases. 2022;27:e01373.
- [CrossRef] [PubMed] [Google Scholar]






