Translate this page into:
Deciphering unilateral congenital facial palsy in neonates: A case series and literature review
*Corresponding author: R. R. Prashanth, Department of Neonatology, Seth GS Medical College and King Edward Memorial (KEM) Hospital, Mumbai, Maharashtra, India. prash2635@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Goyal M, Prashanth RR, Shah S, Mujeeb AA, Mhatre S, Haribalakrishna A. Deciphering unilateral congenital facial palsy in neonates: A case series and literature review. Wadia J Women Child Health. 2023;2(3):116-20. doi: 10.25259/WJWCH_1_2024
Abstract
Unilateral facial palsy in neonatal period either occurs in isolation or in association of a syndrome. We report three neonates, two boys and one girl, who presented with unilateral facial palsy at birth. Clinical assessment was performed by a neonatologist, ear-nose-throat (ENT) surgeon, an ophthalmologist and a physiotherapist. Magnetic resonance imaging (MRI) of the brain in two neonates and additional computerized tomography (CT) of the temporal bone in one patient was done to exclude developmental anomalies of the facial nerve. Imaging revealed an underlying underdevelopment etiology with absence of cochlea in one neonate. These findings point towards the importance of imaging and systematic diagnostic work-up. This article describes a multidisciplinary approach toward unilateral, isolated congenital facial palsy along with a literature review. Role of physiotherapy, auditory rehabilitation, and other medical and surgical options have also been discussed.
Keywords
Congenital
Facial nerve
Injury
Neonatal intensive care units
Diagnosis
INTRODUCTION
Facial nerve palsy in neonates usually occurs following instrumental delivery or due to abnormal development of the facial nerve either in isolation or as part of a syndrome. The earliest reported case of facial palsy in newborn dates back to 1951; however, the management principles in the neonatal period require further inquisition.[1] This study describes three cases of neonatal unilateral facial nerve palsy, with varied etiologies, over a period of 6 months from July 2022 to December 2022 in a tertiary level neonatal intensive care unit (NICU).
CASE SERIES
CASE 1
A 24-year-old multigravida with no antenatal risk factors delivered, through vacuum extraction secondary to prolonged second stage of labor, a female neonate at term gestation with birth weight of 3025 grams with APGAR scores of 9 at both 1 and 5 minutes. Examination soon after birth revealed deviation of angle of mouth to the right side while crying and inability to close the left eye completely. In addition, there was also absence of the left-sided nasolabial fold and reduced sucking, which suggested a left-sided congenital facial nerve palsy (lower motor neuron type) [Figure 1a]. The neonate was referred to us at 3 hours of life for further management. On revisiting history, there was difficulty in extracting the shoulders. There was no application of forceps. Further examination revealed a head circumference of 34 cm. House-Brackmann facial nerve injury grading scale at the time of examination was 16. There was no evidence of dysmorphic features, birth trauma, focal neurological deficit, and rest of the physical and systemic examination was normal.
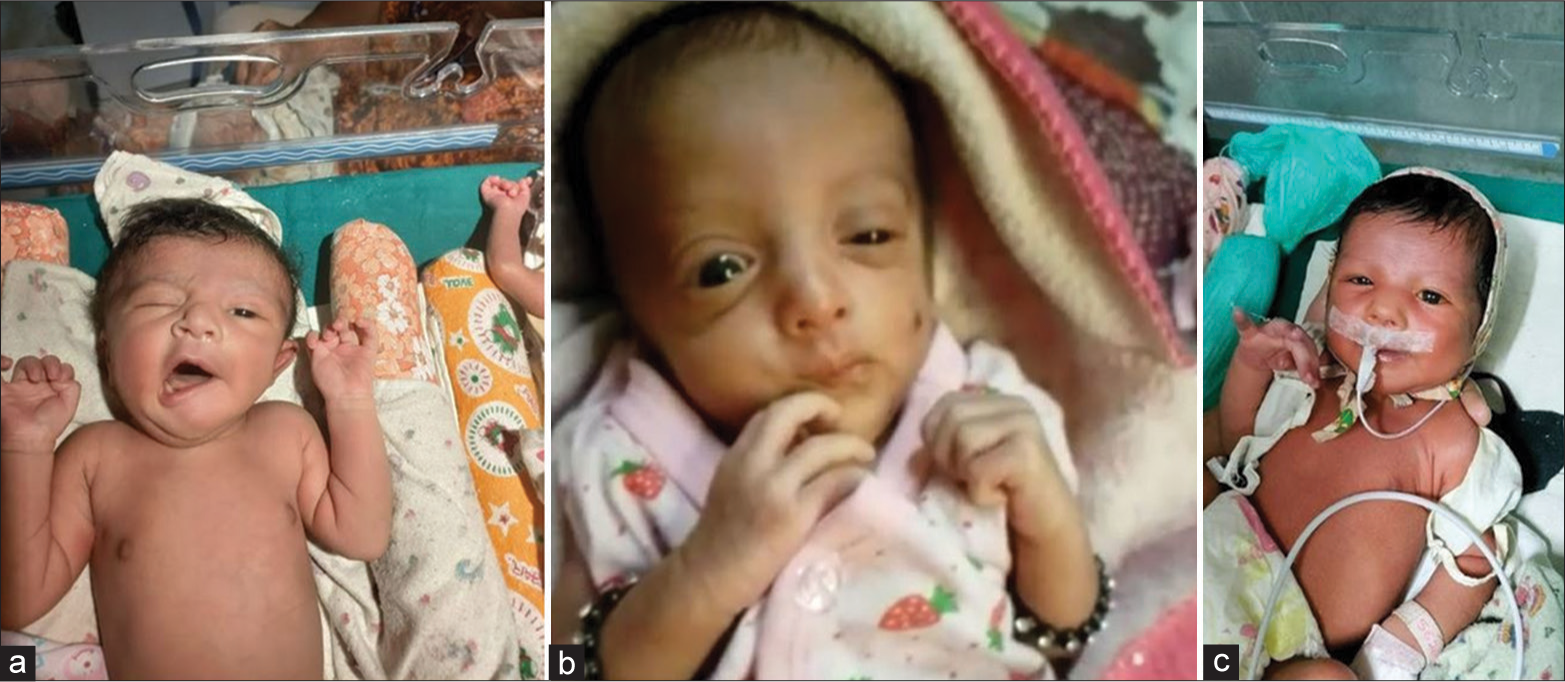
- (a) Case 1 with deviation of angle of mouth to the right side while crying, inability to close the left eye completely, absence of the left nasolabial fold suggestive of the left lower motor neuron congenital facial palsy; (b) Case 2 with deviation of angle of mouth to the left side while crying, inability to close the right eye completely, absence of the right nasolabial fold suggestive of the right-sided lower motor neuron congenital facial palsy; and (c) Case 3 with deviation of angle of mouth to the right with incomplete closure of the left eye suggestive of the left lower motor neuron facial palsy.
On further evaluation, the tympanic membrane and indirect ophthalmology were normal. She was initiated on oromotor exercises and was fed expressed breast milk through cup and spoon. There was gradual improvement in facial muscle function and she was discharged by day 10 of life. On subsequent follow-up, there was marked clinical improvement with complete resolution by 3rd week of life. Audiological screening with otoacoustic emissions (OAE) was normal. However, further neuroimaging and brainstem-evoked potential (BERA) could not be done as she was lost to follow-up.
CASE 2
A 26-year-old multigravida delivered a male infant at 32 weeks gestation with a birth weight of 1898 grams after spontaneous onset of preterm labour. The infant was delivered in breech position. He had APGAR scores of 9 at both 1 and 5 minutes and was noted soon after birth to have deviation of angle of mouth to the left side while crying and inability to close the right eye completely, suggestive of the right-sided lower motor neuron type facial palsy [Figure 1b]. There was no history of difficult extraction, application of forceps, or vacuum. Examination revealed a head circumference of 33 cm and House-Brackmann facial nerve injury grading scale was 20. There was abnormality of the left pinna with abnormal tragus, but the rest of the examination was normal. The neonate was under multidisciplinary management and audiologic assessment with OAE was normal. Indirect ophthalmology showed marked pallor of optic disc. He was initiated on physiotherapy for facial muscle weakness by day 4 of life. Baby was discharged on day 16 of life on cup and spoon feeding and facial nerve grading scale score persisted to be 20, suggesting no improvement. Neuroimaging, which was done to rule out structural defects, was normal.
CASE 3
A 28-year-old delivered vaginally, a male neonate at 39 weeks gestation with birth weight of 3598 grams. The neonate had APGAR scores of 9 at both 1 and 5 minutes, and no resuscitation was required. Examination soon after birth revealed deviation of angle of mouth to the right with incomplete closure of the left eye suggestive of the left lower motor neuron facial palsy [Figure 1c]. On revisiting history, the neonate was born of third-degree consanguineous marriage and an antenatal scan was suggestive of bilateral prominent renal pelvis (5.2 mm). There was no history of difficult extraction or application of forceps or vacuum. Further, examination revealed a head circumference of 33 cm and House-Brackmann scale was 20.
Postnatal renal ultrasound was suggestive of the right-sided duplex ureter moiety with moderate hydroureteronephrosis due to vesicoureteral reflux with pyonephrosis. Renal function tests (blood urea nitrogen 10.7 mg/dL and creatinine 0.7 mg/dL) and urinalysis with culture were normal. However, the baby had multiple episodes of multifocal clonic seizures on day 7, 8, and 13 of life. A video electroencephalogram was normal. Metabolic workup showed low ionized calcium (0.78 mmol/L) and normal blood glucose, electrolytes, and serum magnesium. Lumbar puncture was normal and blood and cerebrospinal fluid cultures were sterile. Magnetic resonance imaging (MRI) brain with temporal bone showed a normal brain study with a right inner ear malformation [Figure 2]. Computed tomography (CT) temporal bone showed common cavity malformation of the right ear with absent superior and lateral semi-circular canals along with an atretic internal auditory canal [Figure 3]. BERA corroborated with the CT and showed right-sided profound hearing loss (Pure-tone audiometry >105 dB hearing loss). The ophthalmological evaluation showed incomplete left eye closure. Echocardiography showed a small atrial septal defect with left to right shunt.
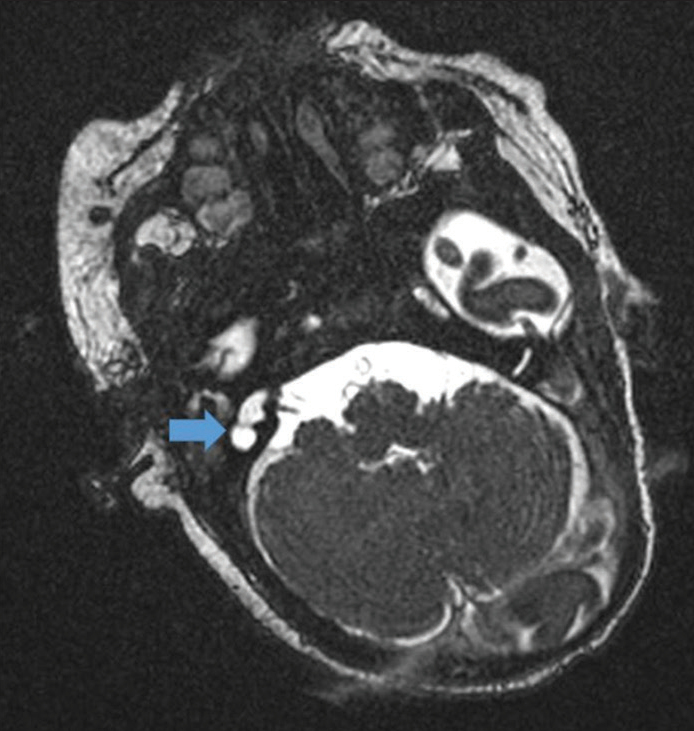
- Magnetic resonance imaging (MRI) brain with cochlear cuts in (case 3) showing normal brain study with the right inner ear malformation (marked with blue arrow).
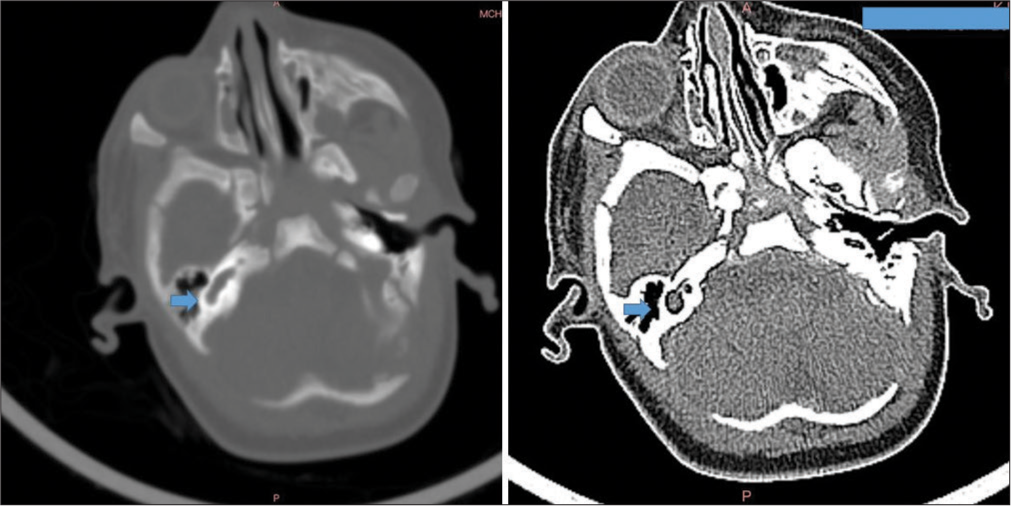
- High-resolution computed tomography temporal bone showing confluence of cochlea and vestibule into a common cystic cavity with no internal architecture with deformed posterior semicircular canal and non-visualization of superior and lateral semicircular canals (marked with blue arrows).
In view of incomplete left eye closure, the baby was given carboxymethylcellulose eye drops 4th hourly with eye padding. He was also initiated on early intervention therapy for axial and appendicular hypotonia and physiotherapy for facial nerve palsy. After the ear-nose-throat consult, BERA was planned to be repeated after 3 months to decide on cochlear implantation (on the better ear followed by worse ear). Until then, the baby is planned to be given hearing aids to assist with the rehabilitation. On follow-up at 6 weeks, facial nerve deficits persisted, House-Brackmann score was 20 and he also had axial and appendicular hypotonia. In view of the inner ear abnormality, renal anomaly, and left-sided lower motor neuron facial nerve palsy, the possibility of branchiootorenal syndrome was considered; however, genetic confirmation was not pursued in view of financial constraints.
DISCUSSION
Congenital facial palsy has been reported 0.2–6.9% of all live births.[2] In nearly 90% of cases birth trauma is the most common cause, of which 90% cases are following forceps application.[3] However, in a retrospective study done in 2010, only 24% had a history of forceps application.[4] In the absence of a history of difficult extraction, compression of the face against the maternal sacral promontory or ischial spine during birth along with a partially developed mastoid process in neonates, especially preterm, possibly impacts the facial nerve as it exits the stylomastoid foramen.[4] This risk increases with lower gestational age, during prolonged labor and augmentation of labor with oxytocin.[5] This could possibly explain the facial palsy in the second case, as the baby was preterm and delivered by a breech extraction.
Moebius, Poland, Goldenhar, Branchiootorenal (BOR), cardiofacial, CHARGE syndromes, hemifacial microsomia, and Arnold–Chiari disorder are other conditions associated with peripheral facial palsy.[6] Abnormalities of external as well as inner ears are also associated with facial nerve palsy. Common cavity malformation is a deformity in which the cochlea and the labyrinth form one space. A review by Coudert et al., in 2019, found that inner ear malformations were associated with a facial nerve anomaly in 11% of cases.[7] Association between inner ear malformation and facial nerve anomaly ranges from 4% to 66.5% in various studies.[7] One such association is the branchiootorenal syndrome (BOR). To diagnose BOR, major criteria include branchial anomalies, deafness, preauricular pits, or renal anomalies and minor criteria include inner, middle, and external ear anomalies, preauricular tags, facial asymmetry, and palatine abnormalities. In our review, case three had two major features (deafness and renal anomalies) and two minor anomalies (inner ear anomalies and facial palsy) suggesting BOR.[8]
Manifestations of lower motor facial palsy can range from difficulty in closing the eye, absence of forehead wrinkling, absence of nasolabial fold and asymmetry of the face especially on crying with deviation of angle of mouth toward normal side to complete absence of facial movements on the affected side. There may be an impairment in sucking. During physical examination, it is important to differentiate lower motor facial palsy from upper motor facial palsy. A lower motor (peripheral) facial palsy results from a lesion at the root entry zone of brainstem or more distally along the course of facial nerve in the middle ear or face and results in ipsilateral muscle weakness involving both upper and lower part of the face. In addition, the nerve also has sensory and visceral functions. Therefore, deficits are often accompanied by lagophthalmos, hyperacusis, change in taste (anterior two-thirds of tongue), or increased tear production, which are slightly difficult to ascertain in the neonatal period. The physical examination should also focus on other associations including abnormality of external ears, features of traumatic delivery including periauricular or facial ecchymosis, or hemotympanum and dysmorphic features.
The objective assessment of the extent of facial nerve palsy in neonates is often done by House-Brackmann scale, which is extrapolated from adults and requires validation in newborns.[9] It is important to validate these scales to allow objective quantification of the extent of facial muscle involvement, and guide subsequent physiotherapy and follow-up.
There is no role for routine imaging in neonates with traumatic facial nerve palsy. Indications of MRI include no improvement by 3 months in a case of traumatic facial nerve palsy, associated syndromic association such as CHARGE or BOR syndrome, a suspected upper motor neuron facial palsy, or bilateral facial palsy.[10] Imaging of choice is MRI with a three-dimensional constructive interference steady state sequence.[11] Complete course of facial nerve from the brainstem, till parotid should be traced. CT temporal bone imaging, as in our case, can give additional information about the facial canal and the presence of inner ear abnormalities. Similar to our case, in BOR syndrome, malformations of the ossicles and middle ear cavity have been described, such as hypoplastic apical turn of the cochlea (incomplete partition type II or “Mondini dysplasia”), a facial nerve medial to the cochlea, a funnel shaped internal auditory canal, and a patulous eustachian tube. As in our neonate, the presence of an “unwound cochlea” on imaging has also been reported specifically in patients with BOR.[7,12]
Most of the traumatic lower motor facial nerve palsy in a newborn resolves by 3–6 months, sometimes by 2 years of age without intervention. Poor prognostic factors in traumatic facial nerve palsy include unilateral complete paralysis at birth, hemotympanum, displaced temporal bone fracture, absence of voluntary and evoked motor unit responses by 3–5 days of life, and no improvement of facial nerve function by 5 weeks of age.[13] Physiotherapy and rehabilitation in neonatal facial paralysis is debatable, especially in those occurring secondary to traumatic delivery. As there are no major functional problems during the neonatal period secondary to unilateral facial nerve palsy, an aggressive therapy is often not sought and watchful waiting is done till 3 months of age. It is always important to look for spontaneous facial movements and keep the baby on follow-up and ask for parents to continue observing. The use of corticosteroids for accelerating facial palsy recovery has not shown consistent benefit, despite the theoretical role of inflammation and edema in pathogenesis.[14]
Other treatment goals include the prevention of dryness of eyes by artificial tears and eye padding. Audiological rehabilitation in BOR syndrome includes middle ear surgery with cochlear implantation. In cases with permanent facial palsy, such as those following developmental dysplasia, reconstruction of the facial muscles by plastic surgeons can be done.
CONCLUSION
Isolated congenital facial nerve palsy is often associated with birth trauma or syndromes.
Aggressive physiotherapy, rehabilitation of vision and hearing, early initiation of breast feeding helps in optimal growth and development.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Facial paralysis and muscle agenesis in the newborn. Arch Otolaryngol. 1969;89:131-43.
- [CrossRef] [PubMed] [Google Scholar]
- Neonatal asymmetric crying facies: A new look at an old problem. Clin Pediatr (Phila). 2005;44:109-19.
- [CrossRef] [PubMed] [Google Scholar]
- Facial nerve palsy in the newborn: Incidence and outcome. Plast Reconstr Surg. 1990;85:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- Traumatic facial nerve palsy in newborns: Is it always iatrogenic? Am J Perinatol. 2010;27:711-3.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital unilateral facial paralysis. Pediatrics. 1996;97:261-4.
- [CrossRef] [PubMed] [Google Scholar]
- Developmental unilateral facial palsy in a newborn: Six cases and literature review. Eur J Pediatr. 2020;179:367-75.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of inner ear malformations associated with a facial nerve anomaly in 653 children fitted with a cochlear implant. Clin Otolaryngol. 2019;44:96-101.
- [CrossRef] [PubMed] [Google Scholar]
- Branchiootorenal spectrum disorder In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, eds. GeneReviews. Seattle, WA: University of Washington Seattle 1993; 2023. 1999
- [Google Scholar]
- Facial nerve grading system 2.0 Otolaryngol. Head Neck Surg. 2009;140:445-50.
- [CrossRef] [PubMed] [Google Scholar]
- CT and MR imaging of the normal and pathologic conditions of the facial nerve. Eur J Radiol. 2001;40:133-46.
- [CrossRef] [PubMed] [Google Scholar]
- Magnetic resonance imaging of developmental facial paresis: A spectrum of complex anomalies. Neuroradiology. 2018;60:1053-61.
- [CrossRef] [PubMed] [Google Scholar]
- The unwound cochlea: A specific imaging marker of branchio-oto-renal syndrome. AJNR Am J Neuroradiol. 2018;39:2345-9.
- [CrossRef] [PubMed] [Google Scholar]
- Management of facial palsy caused by birth trauma. Laryngoscope. 1986;96:381-4.
- [CrossRef] [PubMed] [Google Scholar]
- Corticosteroids for bell's palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2016;7:CD001942.
- [CrossRef] [PubMed] [Google Scholar]






