Translate this page into:
Efficacy of diclofenac transdermal patch versus injection as post-cesarean analgesia: A clinical, comparative, single-blind randomized control trial
*Corresponding author: Mohan Sunkad, Department of Community Medicine, USM-KLE-International Medical Program, Belgaum, Karnataka, India. dsmohan_s@rediffmail.com
-
Received: ,
Accepted: ,
How to cite this article: Dhamankar S, Javali SB, Sunkad M. Efficacy of diclofenac transdermal patch versus injection as post-cesarean analgesia: A clinical, comparative, single-blind randomized control trial. Wadia J Women Child Health. 2024;3:61-7. doi: 10.25259/WJWCH_26_2024
Abstract
Objectives:
Post-operative pain is a common problem following cesarean delivery. The efficacy of topical application of diclofenac in alleviating pain has not been well established. We aim to evaluate the analgesic effect of transdermal diclofenac patch and compare it with the injectable form.
Material and Methods:
This is a prospective, randomized, single-blind, and placebo-controlled study. The study protocols and ethical considerations were adhered fully. Parturient women (n = 90) undergoing cesarean section were randomized into two equal groups: the transdermal diclofenac patch (Group A) and the injectable diclofenac (Group B). The surgery was performed under spinal anesthesia. The participant’s demographic data, clinical data, pain relief data collected, compiled, and analyzed using the Statistical Package for the Social Sciences 20. The post-operative pain was scored using Visual Analog Scale. Pain relief scores were compared between the groups using Mann–Whitney U-test and Wilcoxon matched-pair test.
Results:
The participants match in demographic and clinical characters. The pain relief scores at 12 h was – 3.10 ± 0.74 and 3.04 ± 0.21 respectively while at 24 hours was – 2.48 ± 0.51 and 2.09 ± 0.29 respectively in Group A and Group B. The difference between Group A and Group B was statistically significant (P = 0.0022). The pain relief scores in both groups match was statistically significant at 6–24 h and 6–48 h (P = 0.0001, 0.0001).
Conclusion:
Diclofenac transdermal patch gives equal pain relief as that of routine injectable formulation.
Keywords
Diclofenac
Transdermal
Cesarean delivery
Post-operation pain
INTRODUCTION
Cesarean deliveries are quite common in obstetric practice. The procedure does cause severe acute tissue injury and results in unbearable pain. An adequate pain relief after cesarean section encourages early movement and will help provide better care of the baby.[1]
Lower segment cesarean section (LSCS) is a class one surgical procedure, where surgical incision, cutting, separation, and access to the surgical site are cleaner with no bacterial contamination. The procedure involves opening the tissues, retracting the layers, clamping the cut blood vessels, tying the knots, and suturing the cut layers, and all these events damage the cells, stimulate the nerve endings, trigger liberation of leukotrienes, and signal the formation of prostaglandins to mitigate the tissue injury. In this scenario, natural physiological response is the unpleasant feeling of pain at the site of operation.
The intensity of pain is influenced by age, personality, cultural background, and current health status of the mother.
The management of post-operative pain is a critical component in patient care activity and we now know the pain biology better and appreciate the pain cascade of injury like, cellular damage, leukotrienes, cyclooxygenase and formation of prostacyclin, prostaglandins and accordingly suitable pharmacologic agents to block this pathway, have heralded the innovation of non-steroidal anti-inflammatory drugs (NSAIDs). The drug diclofenac is an NSAID. The routine use of intravenous infusion of paracetamol for post-operative analgesia is also well known.
It is agreed that the choice of analgesia should be effective and safe, without interfering the mothers’ ability to take care of her baby along with no adverse effects to the baby.
The drug diclofenac is one such NSAIDs. Oral and injectable diclofenac has been used widely for postoperative pain relief.[2-4] Oral administration is the preferred choice in daily practice, but becomes impractical before and soon after surgery. The drug acts primarily by blocking the cyclooxygenase and lipoxygenase enzymes, thus halting the inflammatory pathway and providing relief from pain. When given orally, the drug is readily absorbed, however, only 50% reaches the circulation due to first-pass metabolism. However, when given intramuscular/intravenous, the drug quickly reaches the optimum blood levels. However, these are invasive routes. Newer modes of drug delivery systems are now available with the understanding of pain pathophysiology and treatment, and new routes of drug delivery are being used, with the objective of attempting to block pain at peripheral sites, with maximum active drug and minimal systemic effects. The result is availability topical (transdermal) preparations. The topical preparations being non-invasive in nature, simple to use, assuring steady plasma concentration, and giving therapeutic effect are an added benefit. During transdermal drug delivery system, the drug passes through intercellular spaces at the epidermis by its amiable physical, chemical, pharmacological, and drug dynamic characters, absorbed into the circulation through dermal capillaries. The absorption is steady, reaches optimum therapeutic level by a few hours, and lasts 24 h. Finally, the drug is eliminated by oxidative metabolism in the urine.
The use of transdermal diclofenac patch for pain relief in acute tissue injury following tooth extraction, dental implants, and dental surgeries is well studied and documented.[5-13] There is also good number of studies showing efficacy of transdermal diclofenac patch in osteoarthritis, acute injuries, abdominal laparoscopic surgeries, and herniorrhaphy procedure.
However, there is not enough literature available on the application of transdermal patch as post-operative analgesia in LSCS cases.
This is the focus of our study. We evaluated and compared the analgesia obtained between transdermal diclofenac patch with intramuscular diclofenac injection in patients undergoing LSCS.
MATERIAL AND METHODS
We obtained approval from our Institutional Ethical Committee (Ref: USM KLE/01 20/Oct 3, 2022), for our study and registered with Clinical Trial Registry India (CTRI/2022/12/048088). This is a hospital-based, prospective, randomized, comparative study conducted in the Obstetric Gynaecology department of a Teaching hospital. The CONSORT guidelines are adopted for reporting the study.
Inclusion criteria
All parturient women, between the ages of 18 and 40 years, undergoing LSCS at term after a healthy, normal pregnancy, with, no or mild systemic diseases, satisfying to the American Society of Anesthesiologists (ASA) Group I and II, who are willing to participate are included in the study.
Exclusion criteria
Patients unsuitable for spinal anesthesia, history of allergy to analgesic drugs, women with bowel disorders like ulcers, inflammatory bowel disease were excluded from the study.
A group of 90 pregnant women were thus randomly divided into group A (treated with diclofenac transdermal patch) and group B (treated with injectables) of 45 women each.
The sample size calculation was based on the frequency of cesarean deliveries, the pregnant women satisfying inclusion criteria, matching the groups with demographic data, random number sequencing, allocation to groups, blinding of study by Statistician. The appropriate medicines were put in sealed, labeled individual envelopes.
A written informed consent was taken from all the participants, then detailed them of probable side effects and collected demographic information (age, gender, education, occupation, body mass index, height and weight, deleterious habits [smoking/alcohol] and ASA status) of the patients was recorded. Detailed medical history and thorough physical examination with routine blood investigations were done to assess and investigate any preexisting or pregnancy related metabolic or systemic condition. Baseline pulse rate, blood pressure, respiratory rate, and oxygen saturation values were taken as routine investigation.
Postoperatively, analgesic was given in the 1st hour (h). Group A was given transdermal patch with 1 cc of normal saline injection (placebo) intramuscularly and group B received injectable diclofenac with non-medicated patch (placebo).
The medicated diclofenac patch and non-medicated placebo patch, measure 10 cm * 14 cm, are applied on the back, subscapular area. The patch is replaced after 24 h.
Pain monitoring was done every six hourly for 2 postoperative days with visual analog six-point scale. In pain research, the Visual Analog Scale (VAS) is the most common tool used. The tool has a line with a begin point “No Pain” and “End Point” worst imaginable pain [Table 1].
Visual analog pain scale
| No pain | Mild pain | Moderate pain | Severe pain | Very severe pain | Unbearable pain |
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 |
The parameters such as onset of pain postoperatively, dose of medicine (transdermal or injectable dose) duration of action, the dose of additional rescue analgesia, adverse effects of transdermal patch, and injectable were recorded.
Rescue analgesic
In the event of visual analog score more than four (severe pain), 100 ml IV infusion of paracetamol was given as rescue analgesia.
A team of nurses and assistants were enlisted and trained for the role expected. The entire study was accurately documented.
RESULTS
Enrolled 95 participants, 5 participants excluded for short of inclusion criteria.
The “Flow Chart” [Figure 1]:

- Flowchart.
We finalized 45 symmetrical pairs of participants for the study, matching the demographic characters.
The mean age of participants is 27.20 ± 5.10 years. The participants are evenly distributed across the sociodemographic characters [Tables 2-4].
| Profile | Transdermal group | % | Injectable group | % | Total | % | Chi-square | P-value |
|---|---|---|---|---|---|---|---|---|
| Age groups | ||||||||
| ≤25 years | 16 | 35.56 | 23 | 51.11 | 39 | 43.33 | 2.5810 | 0.2750 |
| 26–30 years | 22 | 48.89 | 15 | 33.33 | 37 | 41.11 | ||
| ≥31 years | 7 | 15.56 | 7 | 15.56 | 14 | 15.56 | ||
| Mean age | 27.20 | 25.73 | 26.47 | |||||
| SD age | 5.18 | 3.85 | 4.60 | |||||
| Education | ||||||||
| Lower | 2 | 4.44 | 0 | 0.00 | 2 | 2.22 | 4.9090 | 0.0560 |
| Middle | 35 | 77.78 | 42 | 93.33 | 77 | 85.56 | ||
| Higher | 8 | 17.78 | 3 | 6.67 | 11 | 12.22 | ||
| Occupation | ||||||||
| Housewife | 40 | 88.89 | 44 | 97.78 | 84 | 93.33 | 4.1900 | 0.1230 |
| Nurse | 4 | 8.89 | 0 | 0.00 | 4 | 4.44 | ||
| Others | 1 | 2.22 | 1 | 2.22 | 2 | 2.22 | ||
| Total | 45 | 100.00 | 45 | 100.00 | 90 | 100.00 | ||
SD: Standard deviation
| Parameters | Transdermal group | Injectable group | t-value | P-value | ||
|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | |||
| Height | 154.42 | 7.67 | 153.28 | 5.73 | 0.6844 | 0.4961 |
| Weight | 64.19 | 8.94 | 64.06 | 14.48 | 0.0448 | 0.9644 |
| BMI | 26.95 | 3.52 | 27.38 | 5.16 | −0.4077 | 0.6848 |
BMI: Body mass index
| Parameters | Transdermal group | Injectable group | t-value | P-value | ||
|---|---|---|---|---|---|---|
| Mean | Std. Dev. | Mean | Std. Dev. | |||
| Pulse rate | 84.62 | 6.70 | 83.11 | 4.01 | 1.2981 | 0.1976 |
| Respiratory rate | 20.02 | 2.54 | 20.53 | 2.42 | −0.9785 | 0.3305 |
| SPO2 | 99.60 | 0.54 | 99.64 | 0.53 | −0.3947 | 0.6941 |
| Temperature | 98.15 | 0.69 | 97.70 | 0.62 | 3.2657 | 0.0016* |
| SBP | 116.68 | 8.17 | 114.59 | 9.34 | 1.1178 | 0.2668 |
| DBP | 74.14 | 6.63 | 73.18 | 6.39 | 0.6876 | 0.4935 |
The clinical characters between the two study groups are equal and matching, summarized in Tables 5 and 6.
| Times | Group A | Group B | U-value | Z-value | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean rank | Mean | SD | Mean rank | ||||
| 6 h | 3.08 | 0.47 | 39.53 | 3.29 | 0.59 | 46.09 | 761.00 | −1.2194 | 0.2227 |
| 12 h | 3.10 | 0.74 | 42.13 | 3.04 | 0.21 | 43.78 | 865.00 | −0.3038 | 0.7613 |
| 18 h | 2.50 | 0.60 | 43.63 | 2.44 | 0.50 | 42.44 | 875.00 | 0.2157 | 0.8292 |
| 24 h | 2.48 | 0.51 | 51.69 | 2.09 | 0.29 | 35.28 | 552.50 | 3.0552 | 0.0022* |
| 30 h | 2.08 | 0.27 | 43.69 | 2.04 | 0.21 | 42.39 | 872.50 | 0.2377 | 0.8121 |
| 36 h | 2.00 | 0.00 | 43.00 | 2.00 | 0.00 | 43.00 | 900.00 | −0.0044 | 0.9965 |
| 42 h | 2.08 | 0.27 | 53.01 | 1.60 | 0.50 | 34.10 | 499.50 | 3.5218 | 0.0004* |
| 48 h | 1.38 | 0.49 | 43.44 | 1.36 | 0.48 | 42.61 | 882.50 | 0.1497 | 0.8810 |
| Groups | Changes from | Mean Diff | SD Diff. | % of effect | Z- value | P- value |
|---|---|---|---|---|---|---|
| Group A | 6 h–12 h | −0.03 | 0.95 | −0.81 | 0.3922 | 0.6949 |
| 6 h–18 h | 0.58 | 0.59 | 18.70 | 4.0145 | 0.0001* | |
| 6 h–24 h | 0.60 | 0.63 | 19.51 | 3.9571 | 0.0001* | |
| 6 h to 30 h | 1.00 | 0.55 | 32.52 | 5.0862 | 0.0001* | |
| 6 h–36 h | 1.08 | 0.47 | 34.96 | 5.3028 | 0.0001* | |
| 6 h–42 h | 1.00 | 0.51 | 32.52 | 5.1594 | 0.0001* | |
| 6 h–48 h | 1.70 | 0.65 | 55.28 | 5.4424 | 0.0001* | |
| Group B | 6 h–12 h | 0.24 | 0.57 | 7.43 | 2.6656 | 0.0077* |
| 6 h–18 h | 0.84 | 0.80 | 25.68 | 4.7821 | 0.0001* | |
| 6 h–24 h | 1.20 | 0.66 | 36.49 | 5.6454 | 0.0001* | |
| 6 h–30 h | 1.24 | 0.65 | 37.84 | 5.7115 | 0.0001* | |
| 6 h–36 h | 1.29 | 0.59 | 39.19 | 5.8413 | 0.0001* | |
| 6 h–42 h | 1.69 | 0.79 | 51.35 | 5.8413 | 0.0001* | |
| 6 h–48 h | 1.93 | 0.72 | 58.78 | 5.8413 | 0.0001* |
The pain scores assessed in both the groups, summarized in Figures 2-4.
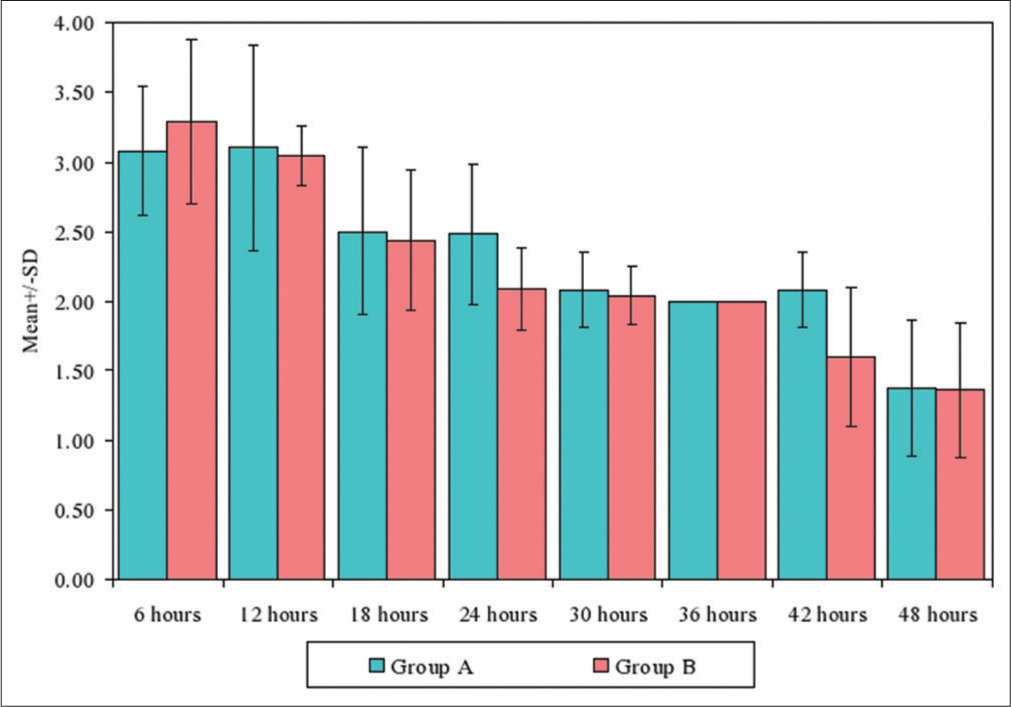
- Comparison of two groups with mean pain scores at different treatment times.
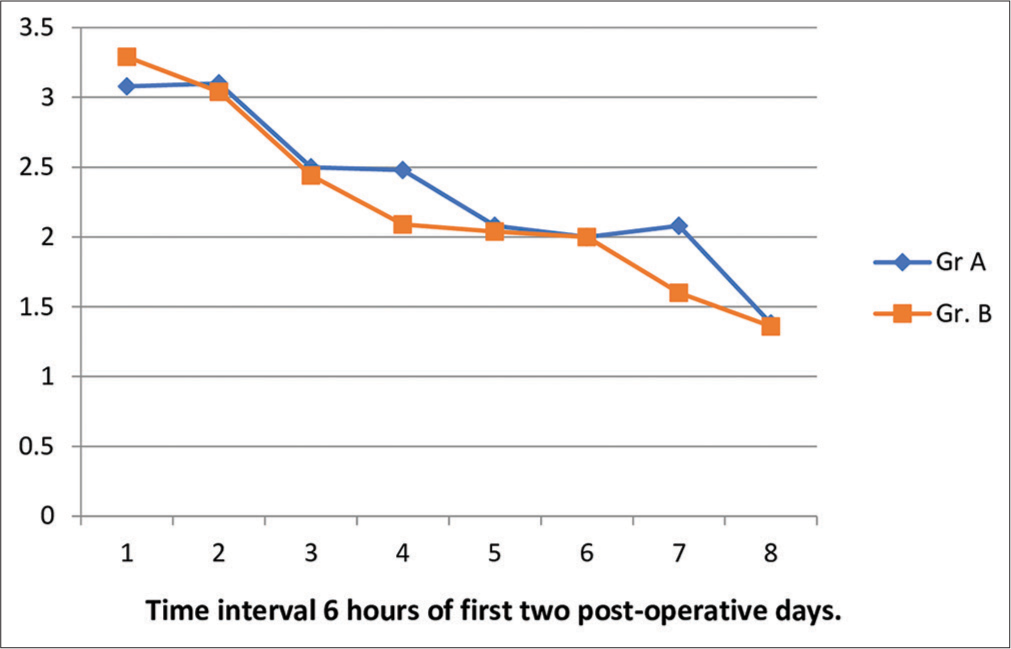
- Trend of visual analog mean pain score of group A and group B.
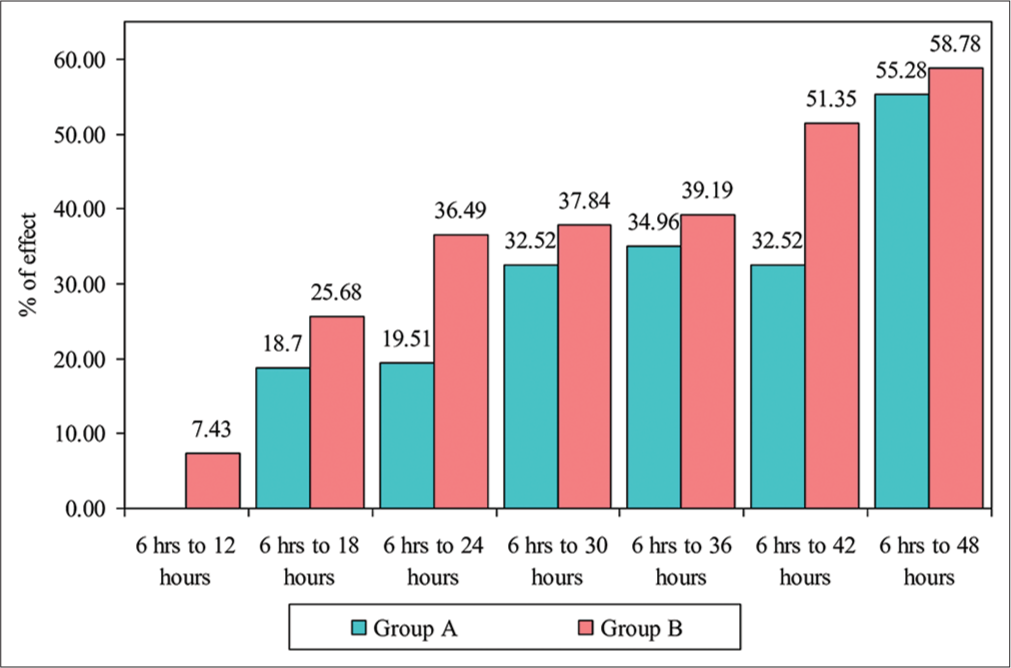
- Comparison of different time points with mean pain scores in group A and group B.
The pain relief scores between Group A and Group B at 12 h was 3.10 ± 074, 3.04 ± 0.21 respectively and the difference was not statistically significant (p>0.05) but at 24 h was 2.48 ± 0.51, 2.09 ± 0.29 respectively which was significant (P = 0.0022).
The pain relief scores in both the groups was statistically significant at 24 h and 42 h (P = 0.0022, 0.0004).
The pain relief scores in both groups match and statistically significant at 6 h–24 h and 6 h–48 h (P = 0.0001, 0.0001).
There are no untoward events recorded in both the groups.
DISCUSSION
The diclofenac formulations are well understood, efficient, safe, and widely used products. However, oral and injectable products are commonly used; the transdermal patch is less commonly used. There is literature mentioning its use for post-operative pain relief.[14-16] In our study, we found good post-operative pain relief using transdermal patch. The quality of pain relief was equal and comparable to injectable diclofenac. In Group A, 43 women the pain relief set in 4 h, lasted 16 h, tapered by 20 h, there was no need for extra analgesics. In two women, pain relief was good but needed extra analgesia after 16 h. In Group B, the pain relief was similar, 42 women had good analgesia, while three women needed extra analgesia after 12 h. The VAS scores in both the groups ranged between one and three in first 12 h. Then, two women in transdermal patch group and three women from injectable group, the score crossed three VAS score. The general condition, clinical parameters, post-operative recovery remained good. There were no untoward events.
There are many studies using transdermal diclofenac patch for cesarean post-operative pain relief. The study by Chawla et al., very systematic, in a sample of 200 mothers, showed excellent analgesia in all the patients. The VAS scores at 6 h, 12 h, 18 h, and 24 h in the 1st post-operation day for both the groups matched evenly in both the groups. This finding is indicative of comparable pain relief action of transdermal patch.[17] In another study, Rajeev and Harikrishnan used diclofenac suppository, transdermal diclofenac patch and control, in 150 women for post-cesarean pain relief, to find excellent analgesia in suppository group, good relief in transdermal patch compared to the control group.[18] Dahl et al. found diclofenac suppository given twice daily, had good pain relief in a sample of 82 mothers.[19] Lim et al., in a sample of 48 mothers, also found diclofenac suppository an excellent pain relief after cesarean delivery.[20]
Both diclofenac suppository and diclofenac patch are locally absorbed and suppository has superior action. All these studies concur with our study results showing comparable pain relief with transdermal diclofenac patch. Perhaps, we need many more such studies from different geoclinical setting, so that the outcomes are really representative.
Further pain relief with diclofenac patch following gynecological procedures and major general surgeries have also showed promising results. The study by Alessandri et al., in a sample of 21 patients undergoing laparoscopic surgery found comparable pain relief with that of standard analgesic.[13]
Kumar et al., in 200 patients found significant pain relief in 65% of patients in the first 24 h using diclofenac patch.[21] In a study by Narzaree et al., also found good analgesic effect of diclofenac patch.[22]
Besides these, numerous studies are using transdermal diclofenac patch in dentistry, orthopedics, and general surgical practice as a useful analgesic. Sharma et al. have found transdermal diclofenac patch useful in mandibular fracture surgery.[23] Singh et al. have demonstrated that the use of transdermal diclofenac patch gives good analgesia in head-and-neck cancer surgery.[24] Samal et al. too have shown transdermal diclofenac providing good post-operative analgesia.[25]
In conclusion, we mention one more study by Bhargava et al., who used transdermal diclofenac patch and injectable diclofenac in 100 women during general surgical procedures, to find excellent analgesia and recommend for routine use of transdermal diclofenac patch.[26]
CONCLUSION
We find that diclofenac transdermal patch gives equal and good pain relief in post-operative period following cesarean delivery.
Ethical approval
The research/study approved by the Institutional Review Board at USM-KLE-International Medical Program, number USM KLE/01 20/Oct 3, 2022, dated October 3, 2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- What is a “clinically meaningful” reduction in pain? Pain. 2001;94:131-2.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of transdermal diclofenac patch as an analgesic following premolar extractions in orthodontic patients. Ann Maxillofac Surg. 2020;10:37-41.
- [CrossRef] [PubMed] [Google Scholar]
- Topical versus systemic diclofenac in the treatment of temporo-mandibular joint dysfunction symptoms. Acta Otorhinolaryngol Ital. 2004;24:279-83.
- [Google Scholar]
- Is a transdermal diclofenac patch better than oral diclofenac tablets: A randomized controlled, trail clinical. Univ J Dent Sci. 2021;7:20-3.
- [CrossRef] [Google Scholar]
- Diclofenac transdermal patch: A potential ingress to maxillofacial surgery. J Maxillofac Oral Surg. 2017;16:170-4.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative evaluation of transdermal diclofenac patch with oral diclofenac sodium as an analgesic drug following periodontal flap surgery: A randomized controlled clinical study. Indian J Dent Res. 2019;30:57-60.
- [Google Scholar]
- Comparative evaluation of transdermal diclofenac patch with oral diclofenac as an analgesic modality following root coverage procedures. Gen Dent. 2014;62:68-71.
- [Google Scholar]
- Comparing the effectiveness of transdermal diclofenac patch and inramuscular diclofenac injecton in postoperative pain relief after inguinal hernia mesh repair: A randomised study in the department of general surgery. J Evid Based Med Healthc. 2015;2:5286-92.
- [CrossRef] [Google Scholar]
- Diclofenac patch for topical treatment of acute impact injuries: A randomised, double blind, placebo controlled, multicentre study. Br J Sports Med. 2004;38:318-23.
- [CrossRef] [PubMed] [Google Scholar]
- A study of efficacy of a single dose of a transdermal diclofenac patch and intramuscular diclofenac-as pre-emptive postoperative analgesia in patients undergoing abdominal hysterectomy. Int J Res Med. 2015;4:96-101.
- [Google Scholar]
- Effects of diclofenac epolamine patch on postoperative sore throat in parturients after cesarean delivery under endotracheal general anesthesia. Acta Anaesthesiol Taiwan. 2009;47:17-21.
- [CrossRef] [PubMed] [Google Scholar]
- Topical nonsteroidal anti-inflammatory drugs for the treatment of pain due to soft tissue injury: Diclofenac epolamine topical patch. J Pain Res. 2010;3:223-33.
- [CrossRef] [PubMed] [Google Scholar]
- Topical diclofenac patch for postoperative wound pain in laparoscopic gynecologic surgery: A randomized study. J Minim Invasive Gynecol. 2006;13:195-200.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of oral diclofenac sustained release versus transdermal diclofenac patch in chronic musculoskeletal pain: A randomized, open label trial. J Pharmacol Pharmacother. 2017;8:166-71.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and side effects of diclofenac patch in treatment of patients with myofascial pain syndrome of the upper trapezius. J Pain Symptom Manage. 2010;39:116-25.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of loxoprofen hydrogel patch versus loxoprofen tablet in patients with knee osteoarthritis: A randomized controlled non-inferiority trial. Clin Rheumatol. 2016;35:165-73.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of the effects of injecting paracetamol and transdermal diclofenac patch as analgesics after cesarean birth. J Obstet Gynaecol Pract POGS. 2023;1:6-8.
- [CrossRef] [Google Scholar]
- Prospective randomised clinical trial study of diclofenac sodium 100mg suppository and trans dermal patch for the duration of post operative analgesia in LSCS patients under going surgery under lumbar subarachnoid block. JMSCR. 2018;6:334-40.
- [CrossRef] [Google Scholar]
- High-dose diclofenac for postoperative analgesia after elective caesarean section in regional anaesthesia. Int J Obstet Anesth. 2002;11:91-4.
- [CrossRef] [PubMed] [Google Scholar]
- Single dose diclofenac suppository reduces post-Cesarean PCEA requirements. Can J Anaesth. 2001;48:383-6.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the role of transdermal diclofenac patch (nupatch) in management of pain in postperative patients. Int J Contemp Med Res. 2017;4:493-6.
- [Google Scholar]
- Efficacy and safety of transdermal diclofenac patch versus intramuscular diclofenac injections in postoperative patients of inguinal hernia. Int J Basic Clin Pharmacol. 2016;5:447-52.
- [CrossRef] [Google Scholar]
- Pre-emptive analgesic efficacy of single-dose transdermal ketoprofen and diclofenac patches in post-operative pain management following open treatment of mandibular fractures: A randomized controlled study. Cureus. 2022;14:e27982.
- [CrossRef] [Google Scholar]
- A prospective case-control study on the comparison of postoperative pain relief with transdermal diclofenacpatch and injection diclofenac. Int J Head Neck Surg. 2015;6:129-33.
- [CrossRef] [Google Scholar]
- Postoperative analgesia with transdermal diclofenac versus intramuscular diclofenac--a comparative study. J Evol Med Dent Sci. 2013;2:3367-76.
- [CrossRef] [Google Scholar]
- Diclofenac patch: A better alternative to injectable diclofenac in postoperative pain management. Int Surg J. 2015;2:623-8.
- [CrossRef] [Google Scholar]







